Have you ever wondered how a once-weekly injection can change the way you manage type 2 diabetes or support weight loss goals? Let’s walk through the Mounjaro (tirzepatide) dosing story together — the safe starting points, how clinicians typically titrate up, and what the research tells us about dose-related benefits and risks. Think of this as a friendly guide you can use to discuss options with your healthcare team.
At a Glance: Mounjaro Dosage
Want the quick version before diving deeper? Here are the essentials you and your clinician will consider when starting Mounjaro.
- Dosing frequency: Given once weekly by subcutaneous injection.
- Typical starting dose: 2.5 mg weekly for the first 4 weeks to help your body adapt.
- Maintenance/titration: After 4 weeks, the dose is commonly increased to 5 mg weekly, with further increases every 4 weeks as tolerated — often progressing through 7.5 mg, 10 mg, 12.5 mg, up to a maximum of 15 mg weekly depending on goals and side effects.
- Why titrate: Gradual increases reduce gastrointestinal side effects and let you assess benefit (A1c lowering and weight change) at each step.
- Monitoring: Check A1c, watch for hypoglycemia if you’re on insulin or sulfonylureas, and report severe abdominal pain, persistent nausea, or other worrying symptoms to your clinician.
If you want a concise, clinician-oriented dosing summary to share with your prescriber, reputable resources like GoodRx’s Mounjaro dosage overview provide practical charts and cost context.
Mounjaro Dosage Overview
How do we translate trial data into real-world decisions? Picture someone named Alex who starts Mounjaro: Alex’s clinician prescribes 2.5 mg weekly for four weeks to reduce initial nausea, then discusses stepping up to 5 mg if tolerated. Every four weeks they reassess blood sugar control, side effects, and personal goals — sometimes stopping at 5 mg for good glucose control, sometimes moving toward 10–15 mg for more weight loss, always balancing benefits and tolerability.
Clinical trials — most notably the SURPASS program — showed that higher tirzepatide doses generally produce greater reductions in A1c and body weight, but also more gastrointestinal side effects. That’s why individualized titration is key: we balance the benefit of additional A1c or weight reduction against tolerability for each person.
- Standard titration schedule (commonly used):
- Weeks 1–4: 2.5 mg weekly (initiation dose)
- Weeks 5–8: 5 mg weekly
- Weeks 9–12: 7.5 mg weekly
- Weeks 13–16: 10 mg weekly
- Weeks 17–20: 12.5 mg weekly
- Week 21 onward: 15 mg weekly (maximum dose)
- When to slow down: If nausea, vomiting, or anorexia occur, your clinician may pause dose increases, extend each step beyond 4 weeks, or reduce supportive meds (e.g., antiemetics) temporarily.
- Drug interactions and hypoglycemia risk: The risk of low blood sugar rises when Mounjaro is added to insulin or sulfonylureas; clinicians often lower those medications when initiating tirzepatide.
Safety notes we’ll both want to keep front of mind: people with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 should avoid GLP-1/GIP receptor agonists like tirzepatide. Cases of acute pancreatitis have been reported with incretin-based therapies, so persistent severe abdominal pain warrants urgent evaluation. Pregnancy and breastfeeding are also times when we avoid Mounjaro due to limited safety data.
Practical tips from clinicians and patients:
- Injection technique: Mounjaro comes in prefilled pens; rotate injection sites (abdomen, thigh, upper arm) and inject once weekly on the same day to build a routine.
- Managing GI side effects: Eat smaller, bland meals, avoid high-fat or spicy foods during dose increases, and stay hydrated. Many people find symptoms lessen after a few weeks at a steady dose.
- Setting expectations: Weight loss and A1c improvements are dose-related and gradual; celebrate early wins like improved fasting numbers or losing a few pounds, and reassess goals with your clinician every month or two.
If you’re researching options and want to compare dosing guidance from another clinical source, the Superdrug write-up on Mounjaro dosing is a useful, patient-focused reference that mirrors the stepwise approach most prescribers follow.
Thinking about medications you might already be taking? It helps to look at how therapies interact. For example, if you’re curious about SGLT2 inhibitors and weight effects, our internal piece Does Jardiance Cause Weight Loss explores that class and can help frame combined-treatment conversations.
Finally, if you’re evaluating treatment options or need a place to find trustworthy pharmacy and clinical resources, consider checking practitioner and patient resources like Coreage Rx for additional guidance and services.
Would you like a printable one-page dosage chart you can take to your next appointment, or a sample conversation script to use with your clinician about titration and side-effect management? I can create either — tell me which would help you most.
What Is the Starting Dose of Mounjaro?
Curious where most people begin with Mounjaro and why the first dose is deliberately small? The usual initiation is 2.5 mg once weekly given by subcutaneous injection for the first four weeks. That low starting dose is not intended to provide the full glucose-lowering effect but to help your body adapt and reduce common gastrointestinal side effects like nausea and diarrhoea.
Think of it like easing into a cold pool rather than jumping straight in — many clinicians prefer this gentle introduction to build tolerance before increasing the dose. Official prescriber guidance that outlines this approach is laid out by the manufacturer; if you want the precise starter recommendations for clinicians, see the guidance on how to get patients started from the manufacturer.
Example: imagine Sarah, recently diagnosed with type 2 diabetes. Her endocrinologist started her at 2.5 mg once weekly for a month; she experienced mild, short-lived nausea that eased within two weeks and then moved up to 5 mg, where she began to see meaningful changes in her blood sugar readings.
What Is the Maintenance Dose of Mounjaro?
Ever wonder what “staying on” Mounjaro looks like once initial tolerability is established? The maintenance dose varies by person and treatment goals, but commonly falls between 5 mg and 15 mg once weekly. After the initial 2.5 mg lead-in, many people are advanced to 5 mg weekly, which is often considered the first therapeutic maintenance dose.
From there, clinicians may escalate every 4 weeks as needed — for example to 7.5 mg, 10 mg, 12.5 mg and up to 15 mg — depending on glycaemic response, weight goals, and tolerability. If you and your clinician are prioritising weight loss as well as glucose control, higher maintenance doses have shown greater effects in trials, but they may also increase the chance of side effects.
For a practical, patient-facing chart that summarises typical dosages and escalation patterns you might find helpful, a clear overview is available from a UK online clinic that reviews Mounjaro dosing approaches explaining common dosing steps. Always coordinate changes with your prescriber so adjustments are safe and targeted to your goals.
What’s the Typical Mounjaro Dosage for Adults?
What do “typical” and “customised” dosing look like in real life? A widely used titration schedule for adults aiming for balance between efficacy and tolerability looks like this:
- Week 1–4: 2.5 mg once weekly (starter dose)
- Week 5–8: 5 mg once weekly
- Week 9–12: 7.5 mg once weekly (if needed)
- Week 13–16: 10 mg once weekly (if needed)
- Optional escalation: 12.5 mg then 15 mg every 4 weeks as tolerated and indicated
Clinical trials (the SURPASS programme) demonstrated a dose-dependent reduction in HbA1c and body weight, which is why some patients end up on 10–15 mg for greater metabolic benefits. At the same time, trials and real-world use show that slower, stepwise increases help many people remain on therapy by minimising GI upset.
Here are two quick real-world vignettes to help you picture it: Miguel started at 2.5 mg and moved to 5 mg after four weeks; his fasting glucose dropped substantially and his clinician kept him at 5 mg as a maintenance dose. Jasmine tolerated the 5 mg but wanted more weight loss and glucose lowering; after discussing risks and benefits she titrated to 10 mg with good results and manageable side effects.
Common concerns include nausea, diarrhoea, changes in appetite, and rare injection-site reactions. Weaving clinical evidence with everyday worries, it helps to know that many people report side effects easing after the first month. If you’re experiencing unusual symptoms (for example, severe abdominal pain or persistent vomiting), contact your clinician promptly. For practical tips on some specific side effects that can come up with incretin therapies, we’ve discussed examples like sulphur burps and how people manage them in another article Sulphur Burps Mounjaro, and our broader Blog has more personal stories and troubleshooting ideas.
Bottom line: start low, titrate slowly, and personalise — that’s the strategy most clinicians use to help you get benefits while keeping side effects manageable. Always discuss dose changes with your prescriber and monitor your blood glucose, symptoms, and any other medications you take so we can keep your plan safe and effective.
What Is Mounjaro’s Form?
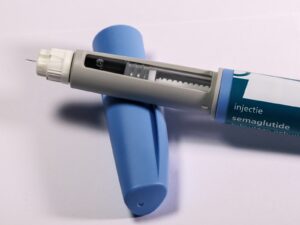
Have you ever wondered what taking a medication like Mounjaro actually looks and feels like in daily life? Picture a slim, prefilled pen—similar to other once-weekly diabetes pens—that you or your caregiver use under the skin (subcutaneously) one time each week. That simple routine is what many patients describe: a short, private moment once a week that can change day-to-day blood sugar management and body weight over months.
How it’s packaged and used: Mounjaro is supplied as a solution in prefilled, single-patient-use pens intended for subcutaneous injection. People often compare the process to using a small insulin pen: choose a site (thigh, abdomen, or upper arm), rotate locations, and administer the dose. Those first few injections can feel like a milestone—some patients describe nervousness at first and then relief at how routine it becomes.
Clinicians and patient reviews often note that the pen format supports adherence because it’s discreet and weekly dosing reduces the daily hassle of multiple medications. If you like reading real-world experiences before a clinic visit, you might find patient perspectives helpful on our Reviews page.
For more technical details about the injectable form, prescribing information and administration guidance are summarized in clinical references and dosing guides.
What Strengths Does Mounjaro Come in?
Curious which strengths are available and why that matters? Mounjaro (tirzepatide) is designed with stepping-stone doses so we can find the balance between effectiveness and tolerability—especially for gastrointestinal side effects that many patients experience early on.
- Available strengths: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg (administered once weekly).
- Typical titration schedule: Most treatment plans begin at 2.5 mg once weekly for four weeks as a starter dose, then increase by 2.5 mg every four weeks until reaching the dose that achieves treatment goals or the maximum of 15 mg weekly. The 2.5 mg dose is primarily a starter dose rather than a long-term maintenance dose.
- Why the step-up matters: Clinical trials (the SURPASS program) have shown that higher tirzepatide doses produce greater reductions in A1c and body weight, but they also tend to bring more GI side effects—so gradual increases are used to improve tolerability while capturing clinical benefit.
If you want a concise clinical summary of dosing options and the recommended titration approach, reliable dosing references outline these strengths and schedules in detail; a commonly used clinician resource is available here: Mounjaro dosing and administration summary.
Think about it like learning to run: we don’t go from the couch to a marathon in a day—small, steady increases help you adapt and reduce the chance of setbacks.
Are There Any Dosage Adjustments for Medical Conditions or Interactions?
Worried about your other health conditions or medications? That’s an important question—because we rarely take medicines in isolation. The good news is that Mounjaro’s dosing pathway is straightforward for many people, but there are some key caveats we should talk about.
- Renal impairment: For most people with mild-to-moderate kidney impairment, specific dose reductions of Mounjaro are not usually required. However, because gastrointestinal adverse effects (nausea, vomiting, diarrhea) can worsen dehydration and kidney stress, close monitoring is sensible in advanced kidney disease.
- Hepatic impairment: Data are more limited for severe liver disease. Severe hepatic impairment calls for caution and individualized clinical judgment; your clinician may choose closer monitoring or alternative therapy depending on liver function.
- Risk of hypoglycemia with insulin or sulfonylureas: If you’re taking insulin or an insulin secretagogue (like a sulfonylurea), adding or increasing Mounjaro can raise the risk of low blood sugar. In practical terms, we often reduce the dose of those drugs when starting tirzepatide and monitor glucose more frequently during titration.
- Effect on oral medication absorption: Mounjaro slows gastric emptying, which can affect the absorption of some oral medications. That means medications that require rapid absorption (for example, certain antibiotics or contraceptives in rare cases) might need timing adjustments or monitoring. Always review essential oral drugs with your prescriber when starting tirzepatide.
- Concomitant incretin therapies: Combining tirzepatide with other GLP-1 receptor agonists is not recommended—there’s no added benefit and potential for increased adverse effects.
- Special populations: In older adults or people who are frail, clinicians typically use slower titration and closer follow-up because of sensitivity to GI side effects and hypoglycemia risk when other diabetic agents are present.
Many of these interaction and adjustment details are summarized in clinical drug references and expert reviews; for a clinician-focused overview of tirzepatide’s pharmacology and safety considerations you can consult a drug monograph such as the one at Medscape: Tirzepatide (Mounjaro/Zepbound) clinical overview.
Gastrointestinal side effects are the most commonly asked-about issue—why do they happen and how long do they last? If diarrhea or nausea is a concern for you, check our explainer on that topic which walks through mechanisms and practical tips: Why Does Mounjaro Cause Diarrhea. In practice, slower dose increases, taking comfort measures (hydration, small meals), and communication with your care team help most people manage symptoms and stay on therapy when it’s appropriate.
Ultimately, dosing adjustments are individualized. Weighing benefits (A1c and weight improvements shown in trials) against tolerability and your overall medication plan is a conversation you and your clinician should have—what matters most is a plan that fits your life and health goals.
What Factors Can Affect My Dosage?
Ever wondered why two people on Mounjaro (tirzepatide) can be on very different doses even though they both have type 2 diabetes? You’re not alone — dosing is a personalized conversation between you and your clinician. Several clinical and personal factors influence the dose you end up taking, and understanding them can help you feel more confident during visits and when making daily choices.
- Baseline blood sugar and A1c goals: If your A1c is far above target, your clinician may aim for a more aggressive titration to reach glycemic goals sooner. Clinical trials like the SURPASS studies showed dose-dependent A1c reductions, which is why doctors tailor intensity to your numbers.
- Weight and weight-loss goals: For people using Mounjaro primarily to lose weight, higher doses tend to produce greater weight loss in trials. That said, we balance benefit with tolerability because higher doses can increase side effects.
- Side-effect profile and tolerability: Gastrointestinal symptoms — nausea, vomiting, diarrhea — are common early on. If you experience persistent effects, your dose may be held or increased more slowly. This is why many clinicians start low and titrate up.
- Kidney, liver function, and age: Organ function and age can affect how we approach dosing and monitoring. Older adults and people with certain comorbidities may need a more cautious plan.
- Other medications and interactions: Drugs that affect gastric motility, or those with overlapping side effects, can change how well you tolerate or respond to Mounjaro.
- Adherence and injection technique: Practical issues matter — missed doses, improper injection technique, or storage problems can all influence perceived effectiveness and the need to adjust dose.
Think of dose selection like tailoring a suit: we’re aiming for a fit that’s effective, comfortable, and sustainable. If you want a clear, patient-friendly overview of the standard dosing strategies and the usual titration schedule, reputable resources like Healthline’s Mounjaro dosing guide provide helpful summaries you can read before your appointment.
Why Would My Doctor Have to Increase My Mounjaro Dose?
Have you ever asked, “If I feel okay on this dose, why increase it?” The short answer: to optimize benefit. Here are the real-world reasons we see clinicians decide to raise a dose.
- Insufficient glycemic control or plateauing effect: If your blood glucose or A1c hasn’t improved as expected after the initial titration window, increasing the dose is a common and evidence-backed step to gain additional A1c reduction.
- Weight loss plateau: Because higher doses of tirzepatide generally produce larger reductions in weight, a dose increase can be considered when weight loss stalls and other strategies (diet, activity) have been optimized.
- Planned, gradual titration to manage side effects: We often start at a low dose — commonly 2.5 mg weekly — to let your body adapt, then step up to 5 mg and beyond at regular intervals as tolerated. These stepwise increases reduce nausea and improve adherence. Clinical summaries and prescribing guidance typically describe this approach in detail; for an accessible review, see Medical News Today’s dosing overview.
- New clinical findings or changing goals: As your overall health, A1c target, or desire for weight reduction changes, your clinician may adjust dose to align with those goals.
- Drug interactions or new medications: Starting other drugs that affect appetite, glucose, or GI transit may prompt dose reconsideration.
One practical example: someone with an A1c of 9% might start at 2.5 mg weekly for 4 weeks, move to 5 mg and, if fasting glucose and A1c haven’t improved enough and side effects are manageable, the provider may continue upward in stepwise fashion to 7.5 mg, 10 mg, and so on — always weighing benefits versus tolerability. Importantly, we never recommend changing your dose on your own; dose adjustments should be guided by your care team and monitoring.
Which Mounjaro Dose Is the Most Effective?
What if we could pick one dose that’s “best”? Here’s the reality: effectiveness is dose-dependent but also person-dependent. Higher doses generally deliver greater reductions in A1c and larger average weight loss in clinical trials, but that doesn’t automatically make the highest dose the best choice for every person.
- Higher doses = greater effect, but more side effects: In randomized trials, escalating doses of tirzepatide showed progressively larger improvements in glucose control and weight. However, nausea and other GI effects also rose with dose. So while 15 mg may be the most potent in a trial, it may not be tolerated by everyone.
- Individual goals matter: If your priority is modest A1c improvement with minimal side effects, a lower dose might be preferable. If maximum weight loss is the main goal and you tolerate therapy well, a higher dose may be discussed with your provider.
- Real-world examples: I’ve seen patients thrive on 10 mg weekly with excellent A1c and steady weight loss, while others ultimately needed 15 mg to break a plateau — and a few preferred to stay at 5–7.5 mg because of side effects. That variability is why personalized plans work best.
- Monitoring and shared decision-making: Choosing the “most effective” dose is less about the number and more about the balance we create together: clinical benefit, side-effect burden, cost, and how the medication fits into your life.
If you’re comparing Mounjaro to other agents like semaglutide, it helps to read clear comparisons so you know how they differ in effect and typical dosing strategies — for instance, our related article Is Semaglutide The Same As Ozempic walks through similarities and differences and can be a useful conversation starter for your next clinic visit. Also, if you use digital portals to manage prescriptions or check dosing instructions, tools like Mochi Health Login can help you keep track of refills and messages from your care team.
Bottom line: there isn’t a universal “most effective” dose for everyone. We aim for the dose that gives you the best mix of benefit, tolerability, and sustainability — and we adjust it as your goals and response evolve. Have you noticed a pattern with your dose and side effects? Bringing that information to your clinician makes titration decisions far more effective and personal.
Mounjaro Dosage Chart and Schedule
Have you ever wondered how doctors decide on the right starting dose for a medicine like Mounjaro (tirzepatide) and how they safely move you up the ladder? You’re not alone — dosing a potent weekly injectable is part science, part art. In simple terms, the goal is to balance rapid improvement in blood glucose (and, for some patients, weight) with tolerability, especially gastrointestinal side effects. Clinical development programs such as the SURPASS trials showed that tirzepatide provides dose-dependent improvements in HbA1c and weight, but those benefits are most safely achieved by gradual titration under medical supervision.
Before we dive into schedules and a practical chart: always treat these schedules as general frameworks. Your prescriber will personalize doses for you based on medical history, current medications, and how you tolerate the drug. If you want a handy external reference, see this comprehensive Mounjaro dosing chart.
Mounjaro Dosage Schedule
So how does the typical schedule actually work? Think of it like learning to run: you start with short, manageable distances to build tolerance and then gradually increase. The standard, commonly used weekly titration sequence looks like this:
- Starter/Initiation phase: 2.5 mg once weekly for 4 weeks — primarily to establish tolerability (this dose is a starter dose and is not intended to be your long-term glucose-lowering dose).
- Titration phase: increase to 5 mg once weekly after the initial 4 weeks; if additional glucose lowering is needed and tolerated, many clinicians escalate every 4 weeks to 7.5 mg, then 10 mg, then 12.5 mg, then 15 mg once weekly.
- Maintenance phase: the dose that achieves your target glycemic control and is well tolerated — many people remain on 5–15 mg weekly depending on goals and side effects.
Why the 4-week intervals? Research and prescribing guidance suggest spacing increases by about a month to allow side effects (especially nausea and GI upset) to settle and for your clinician to assess benefit and safety. Endocrinologists often say: “Titrate slowly to keep you comfortable and on therapy,” which is why the stepwise approach is widely used in practice.
Common practical tips we share with patients: store the pen as instructed, pick a consistent day each week for the injection, rotate injection sites, and keep a symptom log during dose increases so you and your clinician can make decisions together.
The Mounjaro Dosage Chart
Let’s lay out a clear, conversation-friendly “chart” you can use as a reference when discussing options with your clinician. Remember: this is a general template, not a prescription.
- Week 1–4: 2.5 mg once weekly — purpose: acclimate to medication and reduce initial side effects.
- Week 5–8: 5 mg once weekly — purpose: active glucose lowering begins for many people.
- Week 9–12: 7.5 mg once weekly (optional) — purpose: additional HbA1c reduction if needed and tolerated.
- Week 13–16: 10 mg once weekly (optional) — purpose: further titration for greater efficacy in patients who need it.
- Subsequent steps: 12.5 mg then 15 mg once weekly (each step usually separated by ≥4 weeks) — purpose: maximize glycemic and weight benefits in patients who tolerate and require higher doses.
An example scenario: imagine you start at 2.5 mg and after 8 weeks your A1c remains above target but your nausea has settled. You and your clinician might move to 7.5 mg and reassess in 4–8 weeks. That rhythm — try, observe, adjust — is how we safely chase treatment goals together.
What to watch for: nausea, vomiting, diarrhea, decreased appetite, and injection-site reactions are the most common side effects. Rare but important concerns include pancreatitis and a theoretical risk related to medullary thyroid carcinoma; thus, a family history of thyroid cancer or MEN2 is a significant reason to discuss alternatives.
Finally, a few practical and reassuring points: many patients tell us that side effects are most noticeable during initial weeks and during dose jumps, and they often improve over time. If you experience persistent or severe symptoms, contact your provider promptly. Together you can decide whether to pause titration, step back to the prior dose, or try supportive measures (like dietary adjustments or antiemetic strategies).
Would you like a printable version of this chart with space to record dates, doses, and side effects so you can bring it to your next appointment? We can create one that fits your personal goals and timeline.
Understanding the Mounjaro Dose Escalation Schedule
Have you ever wondered why some medicines start small and grow over time instead of beginning at a “full” dose? With Mounjaro (tirzepatide) that stepwise approach is deliberate and crucial. Mounjaro is usually started at a low weekly dose — 2.5 mg — and then increased every 4 weeks to 5 mg, 7.5 mg, 10 mg, 12.5 mg and up to a maximum of 15 mg as tolerated and needed. This schedule comes from the drug’s label and from the way clinicians balance effectiveness with tolerability.
Why that particular cadence? It’s partly pharmacology: tirzepatide is a dual GIP/GLP-1 receptor agonist with a long half‑life that supports weekly dosing, so changes in dose take time to reach steady-state effect. It’s also partly practical: the gradual ramp-up reduces the intensity of common gastrointestinal side effects and lets both you and your clinician judge how well the medication is working before committing to a higher dose.
In the clinical research program (the SURPASS trials) clinicians observed a clear dose-response pattern — higher doses produced greater reductions in blood sugar and body weight — but also increased the likelihood of GI symptoms. That’s why most treatment plans follow a slow, measured escalation to find the sweet spot between benefit and side effects.
- Typical starting dose: 2.5 mg once weekly for 4 weeks (this dose is intended to establish tolerability and is not usually a maintenance dose).
- Usual escalation: increase every 4 weeks to 5 mg, then in 2.5 mg increments up to 15 mg as tolerated and clinically needed.
- Maintenance dosing: varies by person — some stay at 5 or 7.5 mg, others reach 10–15 mg depending on treatment goals and side effects.
Imagine you and your clinician as partners testing volume on a stereo: you turn it up slowly so you can appreciate the sound without blowing the speakers. Similarly, we raise Mounjaro slowly so you can gain benefit while minimizing discomfort.
Why the Dose Increases Gradually
Curious why we don’t start at the highest effective dose right away? The main reasons are safety and tolerability. Have you ever eaten a too-rich meal and felt queasy afterward? That’s similar to what many people experience with incretin therapies when the dose is increased too quickly.
- Reduces gastrointestinal side effects: nausea, vomiting, diarrhea and constipation are the most common adverse effects. Gradual titration lets your gut adapt and often makes these symptoms milder and shorter-lived.
- Balances efficacy and safety: higher doses generally produce larger improvements in A1c and weight, but they also raise the chance of side effects. A stepwise approach helps find the best trade-off for you.
- Limits abrupt metabolic changes: because Mounjaro affects insulin secretion and appetite, slowly increasing the dose helps avoid sudden drops in glucose — especially important if you’re taking insulin or sulfonylureas.
- Based on clinical trial evidence: the SURPASS studies demonstrated dose-dependent benefits and side-effect profiles, which informed the conservative, 4-week titration intervals used in practice.
Experts often compare titration to acclimating to a new climate — if we “move” your system slowly, you’re less likely to experience the shock of rapid change. Practical steps that help during titration include eating smaller meals, staying hydrated, and spacing meals evenly throughout the day. If nausea is a problem, many clinicians recommend simple measures first (ginger, bland foods, slower eating) before considering medications to control symptoms.
Who Decides When Your Dose Increases?
Do you get to decide your dose increase, or does your clinician? The short answer is: it’s a shared decision between you and your healthcare team. We bring the clinical plan; you bring real-world experience of how the medicine feels in your daily life.
- Your prescribing clinician (doctor, NP, PA): typically initiates the schedule and will advise the next steps based on lab results (A1c), home glucose readings, side effects and your overall goals.
- You (the patient): provide essential feedback—how you’re tolerating the medication, changes in appetite or weight, frequency of nausea or GI upset, and any hypoglycemia if you’re on insulin or secretagogues.
- Diabetes educators and pharmacists: often help monitor side effects, teach injection technique, and suggest practical adjustments (timing with meals, symptom management tips).
Here are common scenarios that influence the decision to increase, pause, or even slow titration:
- Good tolerability and persistent hyperglycemia: your clinician will usually proceed with the next step to achieve better glucose control.
- Moderate GI side effects: your clinician may delay the increase or recommend strategies to control symptoms; sometimes the increase is postponed by 2–4 weeks.
- Severe side effects or intolerability: your clinician may stop escalation, reduce the dose, or switch therapies.
- Concomitant insulin or sulfonylurea: because of hypoglycemia risk, these agents often need dose reductions as Mounjaro is increased — decisions are made carefully and usually with more frequent glucose monitoring.
- Special populations: older adults, people with significant gastrointestinal disease, pregnancy or breastfeeding — these situations change the risk/benefit equation and usually prompt more conservative decisions.
Practical tips to be proactive:
- Keep a symptom and glucose log: note how you feel after doses and record glucose readings so your clinician has concrete information.
- Call early if symptoms are severe: don’t wait for the next appointment to report bad nausea, dehydration, or repeated hypoglycemia.
- Ask about medication interactions: some drugs and supplements can change how you respond.
- Discuss goals openly: are we prioritizing A1c reduction, weight loss, or minimizing side effects? That preference guides the pace of escalation.
At the end of the day, titration is a conversation. We plan the steps, monitor the responses, and adjust together so that the dose you land on fits your body and your life. If you’re thinking about starting Mounjaro or are in the middle of a titration, what symptoms or goals do you want to tell your clinician about at your next visit?
What to Expect at Each Stage
Curious about how your body might respond as you move through the Mounjaro dosing steps? Think of the process like learning to run: you start with a slow, short jog and build up as your body adapts. Clinicians use a stepwise titration to balance effectiveness with tolerability, and knowing what typically happens at each stage helps you stay calm and in control.
- Initiation (2.5 mg weekly, usually 4 weeks): Many people start here to give the gut and metabolism time to adjust. Expect mild gastrointestinal symptoms such as nausea, mild constipation or diarrhea, and occasional appetite changes. These often peak in the first week and settle for most patients. In clinical practice, about half of patients report some GI side effects early on, but they commonly improve within 2–4 weeks.
- Early maintenance (5 mg weekly, next 4 weeks): This is when blood sugar effects begin to show more clearly for people using Mounjaro for type 2 diabetes—fasting glucose and sometimes post-meal glucose improve. Weight loss signals often begin to appear. If nausea was present at initiation, it typically lessens; if it persists, providers may hold dose increases or recommend coping strategies.
- Escalation phases (7.5–15 mg, increases every ~4 weeks as tolerated): In the clinical trials in the SURPASS program, higher doses produced progressively larger reductions in HbA1c and body weight. However, higher doses can also increase the chance of GI side effects. Your clinician will weigh glucose control and side effects when deciding whether to progress to 7.5 mg, 10 mg, 12.5 mg, or 15 mg.
- Longer-term maintenance: After reaching a target dose, many people experience steady improvements in glucose control and gradual weight loss over months. Routine monitoring (A1c checks, blood glucose logs, and attention to symptoms) guides ongoing care.
Here are practical, experience-based tips that help most people get through each stage:
- Expect gradual change—your dose likely won’t jump more than once every 4 weeks; that’s intentional to reduce side effects.
- Hydrate and eat small, frequent meals if nausea is a problem—this often reduces intensity.
- Communicate with your clinician—if you’re dizzy, faint, have severe vomiting, signs of pancreatitis, or hypoglycemia (if you’re taking insulin or sulfonylureas), contact them promptly.
An anecdote: one person I know began at 2.5 mg and felt mild nausea for five days, adjusted by eating bland snacks and reducing meal size, and by week three the nausea faded and they noticed less daily snacking. That kind of story is common—initial discomfort followed by meaningful benefit.
Finally, remember the bigger picture: clinical trials (the SURPASS series) demonstrated that tirzepatide improves blood sugar and produces significant weight loss compared with many older therapies, but individual responses vary. We balance effectiveness with comfort, and that’s why the staged approach exists.
How to Take Mounjaro
Want to make the most of your prescription? Taking Mounjaro correctly, consistently, and safely matters—and it’s simpler than it might sound. Let’s walk through timing, storage, missed doses, and interactions in a friendly, practical way.
- Frequency and timing: Mounjaro is given as a once-weekly subcutaneous injection. Pick a day you can consistently remember—many people choose the same weekday, like “every Sunday.” If your schedule changes, you can take the dose up to 48 hours before or after your usual day only when necessary; check with your clinician for exact guidance.
- Starting and titration: Your prescriber typically starts you at a low dose (commonly 2.5 mg weekly) to reduce early side effects, then increases the dose every few weeks as tolerated to reach a therapeutic dose.
- Missed dose guidance: If you miss a weekly dose, take it as soon as you remember within the same week, but if it’s almost time for the next scheduled dose, skip the missed dose—do not double up. Ask your provider for tailored advice because timing nuances can vary by individual circumstances.
- Storage and handling: Before first use, store Mounjaro refrigerated as indicated on the medication label. Keep it away from direct sunlight and do not freeze. After first use, follow manufacturer instructions regarding how long a pen can be kept at room temperature; if unsure, check the label or ask your pharmacy. Always visually inspect the solution—if it looks cloudy, discolored, or has particles, do not use it and consult your pharmacist.
- Medication interactions and safety: If you use insulin or sulfonylureas, combining them with Mounjaro can increase hypoglycemia risk—your insulin or sulfonylurea dose may need adjustment. Inform your team about all medications and supplements. Also be aware of rare but serious risks associated with incretin-based drugs—pancreatitis, gallbladder issues, and a theoretical risk of thyroid C-cell tumors observed in animals—so report severe abdominal pain or persistent vomiting right away.
Practical examples: if you’re traveling across time zones, choose a consistent weekly anchor relative to your home-time preference; if you experience persistent nausea when food is spicy or fatty, try milder meals and smaller portions during the first few weeks.
Finally, bring your pen or dosing record to follow-up visits—tracking weekly doses, side effects, and glucose readings helps your clinician tailor the plan. We’re a team here: your observations guide adjustments.
How to Inject Mounjaro
Nervous about giving yourself an injection? You’re not alone. Many people worry at first, but the injection process is straightforward and quick. Here’s a step-by-step approach that combines clinical best practices and simple tips I’ve seen work in real life.
- Gather supplies: Your Mounjaro pen, a new sterile needle for each injection, an alcohol swab (if you prefer), and a sharps container for disposal.
- Wash your hands: Clean hands reduce infection risk—simple and effective.
- Inspect the pen: Check expiration date and that the liquid looks as described in the leaflet. If anything seems off, don’t inject; call your pharmacist.
- Choose an injection site: The most common sites are the abdomen (except a 2-inch area around the navel), the outer thigh, or the back of the upper arm (if someone else is injecting). Rotate sites each week or each injection to avoid lumps or skin irritation.
- Attach a new needle: Use a new sterile needle every time. Follow the pen instructions to attach and remove needles safely.
- Prime only if instructed: Some pens require priming; follow your specific pen’s leaflet. Many prefilled pens for weekly injectables are dose-specific and may not require priming.
- Administer the injection: Pinch the skin lightly (unless instructed otherwise), insert the needle at the recommended angle (usually 90 degrees for subcutaneous injections), press the button or plunger fully, and hold for the recommended time (often 5–10 seconds) to ensure full dose delivery. Then remove the needle smoothly.
- Dispose of the needle safely: Place used needles in an FDA-cleared sharps container. If you don’t have one, follow local guidance for safe disposal—don’t throw needles in household trash loose.
- Aftercare: If you notice minor redness or a small bruise, it usually resolves quickly. If you see significant swelling, severe pain, or signs of infection, contact your provider.
Here’s a tip many patients find comforting: practice with an empty trainer pen or with a demonstration from a nurse before doing your first full dose. That hands-on rehearsal builds confidence. Also, if you’re afraid of needles, look away during insertion, breathe slowly, and remind yourself the needle is short—most people report minimal pain.
In short, injecting Mounjaro is a repeatable skill you can master. With the right routine, we can minimize discomfort, manage side effects, and focus on the reasons you started this therapy—better glucose control and improving your health. If anything feels unclear, ask your healthcare team to demonstrate the technique in clinic; they’re there to support you.
How Do I Change the Dose on My Pen?
Have you ever stared at a pen and wondered which dial to turn or whether you need a whole new device? Changing the dose on a Mounjaro pen can feel intimidating at first, but once you understand the logic behind the pens and the clinical reasons for gradual increases, it becomes straightforward—and safer.
Start with the prescription and the patient leaflet. The clearest instruction always comes from the label your prescriber wrote and the medication guide in the box. Pens differ: some let you dial an exact weekly dose, while others are single-strength prefilled pens that require switching to a different pen for a new dose. If you’re unsure, call your pharmacist—this single step often saves confusion and errors.
- If your pen has a dose selector: you change the dose by rotating the dial to the prescribed number (for example, from 2.5 to 5 mg). Follow the pen’s instructions for setting the dose, remove the cap, clean the injection site, insert the needle, and activate the injection mechanism. Hold the button as instructed (the patient leaflet will specify the seconds to hold) so the full dose is delivered.
- If your pen is single-strength: you don’t dial a different dose—you exchange the pen for the one that contains the new strength. Never attempt to split or mix doses between pens.
- Always confirm before you change: many clinicians recommend stepping up doses every 4 weeks (to reduce gastrointestinal side effects and allow the body to adapt), but your provider may tailor this to you.
Think of titration like teaching your body to climb a set of stairs rather than leap: experts—endocrinologists and diabetes educators—often emphasize slow, steady increases because clinical trials (for example, the SURPASS program evaluating tirzepatide) show higher doses can improve blood glucose and weight outcomes but also increase GI side effects if advanced too quickly.
Practical example: imagine you—let’s call you Alex—start on 2.5 mg for a month to get used to the medication. After four weeks, your clinician advises increasing to 5 mg. If your pen has a dial, you rotate to 5 mg and inject on your usual day. If it’s a fixed pen, you pick up the 5 mg pen from the pharmacy. If nausea flares when you increase, your clinician might pause at the current dose a little longer.
Key reminders: never split a weekly dose into daily doses, don’t share pens, check the pen’s storage and expiration instructions, and contact your provider before making any change that wasn’t prescribed.
When Is the Best Time to Inject Mounjaro?
When’s the right moment to take a medication you use only once a week? That question matters because the best schedule is the one you’ll stick with—and consistency is where medications like Mounjaro shine.
Make it a weekly habit on the same day each week. Most prescribers recommend choosing a day that fits your routine—Sunday evening, Monday morning, or whatever works—and injecting at approximately the same time each chosen day. The actual time of day is flexible: morning, afternoon, or evening are all acceptable for once-weekly therapies.
Why this flexibility matters: studies and adherence research show that routine and predictability increase long-term use. If you take the dose at breakfast each Sunday, it becomes as automatic as your morning coffee. If you prefer evening, that works too—what matters is the weekly rhythm, not the clock hour.
Practical considerations we talk about as friends and clinicians:
- Travel and time zones: plan ahead—pick a day that will still be that same weekday for you, or consult your clinician if travel crosses multiple days. Many people set reminders on their phone for the chosen day to avoid missed doses.
- Missed doses: policies vary and the manufacturer’s leaflet or your clinician will give the official guidance. If you miss a dose, contact your provider or pharmacist for instructions rather than guessing; dosing windows and safety instructions can be specific.
- Food and other medications: Mounjaro is given subcutaneously and does not need to be taken with meals. However, be mindful when you’re also adjusting other glucose-lowering medications—your clinician may alter timing or doses to reduce overlap and hypoglycemia risk.
Imagine a teacher who always grades papers on Fridays: if you know Friday is grading day, you’ll prepare. Picking a weekly “Mounjaro day” does the same for your medication. If you find your routine changing, we can plan a new steady weekly anchor together.
How Many Mounjaro Pens Will I Need?
How many pens you’ll need isn’t a one-size-fits-all number—it depends on the prescribed dose, the type of pen you receive, how many doses each pen contains, and how long your clinician wants you to stay at each dose during titration. But we can walk through a simple method so you can estimate and plan.
Step-by-step way to calculate your supply:
- Check your prescription for the weekly target dose and the prescribed titration schedule (for example, 2.5 mg weekly for 4 weeks, then 5 mg weekly).
- Look at the pen packaging or the pharmacy label to see how much medicine each pen contains (some pens are adjustable and contain many doses; others are single-strength and each pen is intended for a set number of doses).
- Count how many weekly doses you’ll need for the period you’re planning (4 weeks, 12 weeks, monthly refill) and match that to the doses available per pen.
Illustrative example (hypothetical numbers for explanation only): let’s say your plan is 4 weeks at Dose A, then 8 weeks at Dose B. That’s 12 weekly doses total. If the pens you’re getting each provide 4 weekly doses at the strength you need, you’d need three pens to cover 12 doses. If one of your steps requires a different-strength pen, calculate that portion separately (for example, two pens of Strength A and one pen of Strength B).
There are additional factors to keep in mind:
- Insurance and pharmacy dispensing rules: pharmacies and insurers sometimes fill a 30-day supply or require prior authorization for higher doses, which affects how many pens are dispensed at once.
- Titration changes: if your clinician plans to increase your dose over time, you might receive different pen strengths during your course of therapy—plan for exchanges.
- Practical tip: ask your pharmacist to show you how many doses are in each pen and to do the math with you—pharmacists are great at turning the abstract into exact counts and will tell you exactly how many pens your prescription covers.
Finally, safety-first: never attempt to combine leftover medication from different pens to make a different dose, and never use a pen beyond its labeled number of doses or expiration. If you’d like, bring your prescription details and I’ll help you walk through the numbers together so you know exactly what to expect at pickup or when coordinating refills with your insurer.
How Should I Store My Pens?
Have you ever opened a new pen only to wonder whether you handled it like the medication imagined? Storing Mounjaro correctly is one of the simplest things you can do to protect its effectiveness — and it’s also where small habits make a big difference.
Basic rules: before first use, keep unopened Mounjaro pens refrigerated between 2°C and 8°C (36°F–46°F). Do not freeze the pens. Once you start using a pen, follow the manufacturer’s labeled guidance about how long it can be kept at room temperature; if you choose to store an in-use pen at room temperature, keep it away from heat sources and direct sunlight.
- Keep them in the box while refrigerated to protect from light and accidental knocks.
- Do not freeze — freezing can destroy the protein and make the pen unsafe or ineffective.
- Protect from heat — temperatures above those recommended can degrade the drug quickly.
- Keep caps on to protect the pen and needle from contamination between doses.
- Store out of reach of children and pets and follow sharps-disposal rules for used needles.
Practical example: when traveling, pack unopened pens in an insulated cooler with a cold pack and a thermometer or temperature indicator if possible. For an in-use pen, many people use a small insulated case at room temperature, keeping it in a shaded, cool part of luggage rather than in checked baggage where temperatures can vary widely.
Why this matters: Mounjaro is a peptide-based medication. As with other biologics, temperature and light exposure can change its shape and reduce potency. Experts and regulatory guidance emphasize proper cold-chain handling for peptide medicines — thinking about your pen as you would a delicate food or vaccine helps make the right choices.
Do Mounjaro Pens Expire?
Have you ever held a pen and wondered whether the date printed on the box really matters? Yes — it absolutely does. Mounjaro pens carry an expiration date printed on the carton and often on the pen or label itself.
What the expiration date means: it’s the last date the manufacturer guarantees full potency and safety when the product has been stored under the recommended conditions. Using medication past that date may mean reduced effectiveness or unpredictable performance.
- Unopened pens: use by the printed expiration date when stored as directed (refrigerated, protected from light).
- Opened/in-use pens: the manufacturer also provides a limited window for use after first use (check your package insert or label). Many injectable biologics have a specified number of days (commonly in the range of weeks) after which the in-use pen should be discarded.
- Signs a pen may be compromised: visible discoloration, cloudiness where the liquid should be clear, particles, leakage, or a pen that appears physically damaged — any of these mean you should not use it even if the date hasn’t passed.
Study and expert context: laboratory studies of peptide drugs show that exposure to heat, light, or freezing can cause denaturation or aggregation, which reduces potency and can increase risk of injection-site reactions. That’s why expiration dates and in-use time windows are meaningful, not just bureaucratic stickers.
If you find an expired pen, don’t guess — dispose of it safely and get a replacement from your pharmacy. If you’re unsure whether an in-use pen is still okay, call your pharmacist or healthcare provider; keeping the packaging and noting the lot number helps if you need a replacement or to report a problem.
What Should I Do with a Broken Mounjaro Pen?
We all have those small moments of frustration — a pen drops, cracks, or jams. What do you do if a Mounjaro pen is broken? The short answer: don’t use it, document it, and get a safe replacement.
Immediate steps if a pen is physically damaged:
- Do not inject from a pen that is cracked, leaking, has a broken dose window, or shows any other mechanical failure.
- Safely contain the pen: place it in a puncture-resistant container (a sharps container is ideal) or a rigid plastic container with a secure lid.
- Document the problem: save the carton and label if possible, photograph the damage, and write down the lot and expiration date — manufacturers and pharmacies often ask for this information.
- Contact your pharmacy or prescriber right away to report the broken pen and request a replacement. Pharmacies can guide you about obtaining a new pen and whether the manufacturer will replace it.
- If a partial or no dose was delivered, contact your healthcare provider — do not try to estimate and give an extra dose on your own. They will advise whether to repeat the dose or resume the regular schedule.
Avoid these common mistakes: trying to “fix” a pen, reassembling a dropped needle, or continuing to use a pen that looks compromised. Even if the liquid looks fine, mechanical damage can affect dose accuracy and sterility.
Personal tip: keep a backup pen or two (if your prescriber approves) and a basic travel kit with a small sharps container, alcohol wipes, and instructions for your pharmacist’s contact info. That way, a dropped pen becomes an inconvenience instead of a care interruption.
Finally, if you suspect a manufacturing defect, reporting the issue helps others — manufacturers and regulators track device failures to improve safety. Your pharmacist or provider can assist with that reporting process.
Dosing for Specific Uses and Conditions
Curious how dosing changes depending on why you’re taking Mounjaro and what else is going on in your life? When we talk about dosing for specific uses and conditions, we’re really talking about tailoring a once-weekly plan so it fits your goals, medical history, and daily routine. Clinical trials and practice both emphasize a balance between getting good blood sugar control and minimizing side effects like nausea. Experts often remind patients that a slower, patient-centered approach usually wins: start low, go slow, and check in regularly.
- Why personalization matters: Two people with the same A1c can tolerate different doses because of differences in age, kidney or liver health, other medicines, and how sensitive they are to gastrointestinal effects.
- Conditions that affect dosing decisions: renal or hepatic impairment, history of pancreatitis or gallbladder disease, prior use of GLP-1 receptor agonists, concurrent insulin or sulfonylurea therapy, and personal or family history of medullary thyroid carcinoma or MEN2.
- Shared decision-making: Your clinician will weigh expected benefits (A1c lowering, potential weight loss) against side effects and safety signals. We often revisit dose decisions every 4–12 weeks, depending on how you’re doing.
In practice, you’ll see a common thread: use gradual titration to build tolerance, monitor glucose closely when combining with other glucose-lowering medicines, and stop and evaluate if worrying symptoms (like severe abdominal pain or rapid heart rate) occur. That approach reflects both the trial protocols and what many endocrinologists recommend in clinic.
Dosing Guide for Type 2 Diabetes
Want a clear roadmap for using Mounjaro if you have type 2 diabetes? Let’s walk through a practical guide you can discuss with your clinician. The dosing strategy used in pivotal trials and in prescribing information focuses on a weekly injection with stepwise increases every 4 weeks to balance effectiveness with tolerability. Think of the first few weeks as a gentle cruise-control learning period: we’re getting your body used to the medicine before pushing for maximum benefit.
- Typical titration schedule: Start at a low weekly dose for 4 weeks to reduce GI side effects, then increase every 4 weeks as needed based on glucose control and side effects. This structured ramp-up helps many people stay on therapy.
- Maintenance doses: After titration, several maintenance dose options exist. The goal is to find the lowest dose that gives the A1c and symptom benefit you and your clinician want while keeping side effects manageable.
- How long before we see results? Many people notice improvements in fasting glucose within 1–2 weeks and reductions in A1c by 8–12 weeks. Weight changes may take longer but can be meaningful over months.
- When to reassess: Check A1c every 3 months when changing doses, and sooner if you’re combining therapies that raise hypoglycemia risk.
Remember: dosing for you may differ from what’s typical if you’re taking insulin or sulfonylureas, if you have significant kidney or liver disease, or if you experienced intolerable side effects on another GLP-1 receptor agonist. In those situations, we proceed more cautiously and plan more frequent follow-up.
Dosage of Mounjaro for Type 2 Diabetes
Are you wondering exactly what the labeled dose sequence looks like? Here’s a straightforward outline many clinicians use as a starting point. Keep in mind that individual plans vary, and the starting dose is primarily a strategy to reduce side effects rather than a long-term target for glucose control.
- Starting dose: A low once-weekly dose for the first 4 weeks to help your body adapt.
- Initial escalation: After 4 weeks, increase to the next dose level. This pattern of increasing every 4 weeks continues until we reach the dose that achieves your goals or until side effects limit further increases.
- Available dose levels: Multiple stepwise doses are available; maintenance is typically one of the higher doses after titration. Your clinician will help you pick the right maintenance dose based on A1c targets, weight goals, side effects, and co-medications.
Here are three illustrative examples to make this concrete:
- Example 1 — New to incretin therapy: You start at the low weekly dose for 4 weeks, move to the next level at week 5, and continue stepping up every 4 weeks until your A1c is on target or side effects appear. Many people reach an effective maintenance dose within 2–3 months.
- Example 2 — Switching from another GLP-1 RA: Your clinician may still recommend starting with the low dose or a careful titration schedule to reduce GI symptoms; we don’t assume a direct dose-for-dose switch because individual tolerance varies.
- Example 3 — On insulin or sulfonylurea: Because adding Mounjaro can enhance glucose-lowering, we’ll often reduce insulin or sulfonylurea doses at the start and monitor glucose closely to avoid hypoglycemia. This is a common and important practical adjustment.
Safety notes we always discuss: stop Mounjaro and seek urgent care for symptoms suggestive of pancreatitis (severe persistent abdominal pain), avoid use if you have a personal or family history of medullary thyroid carcinoma or MEN2, and tell your clinician about pregnancy plans—these medicines are not recommended in pregnancy. For kidney or liver disease, clinicians use caution and more frequent monitoring; in many cases mild-to-moderate impairment does not require a dose change, but data are limited in severe disease.
Finally, what do trials and experts tell us? Large trials showed strong A1c reductions and meaningful weight loss with a dose-dependent response. Clinicians emphasize that careful titration and open communication about side effects are the keys to staying on therapy and getting the best outcomes. If you’d like, we can walk through a sample schedule tailored to your current medications and glucose values—would you like to do that now?
How Long Will You Use Mounjaro?
Have you ever wondered whether a medication like Mounjaro is meant to be a short course or a long-term partner? The honest answer is: it depends on why you’re taking it and how you and your provider define success.
For Type 2 diabetes: most people who start Mounjaro (tirzepatide) do so to achieve durable improvements in blood sugar, and stopping the drug often leads to gradual loss of that benefit. Clinical experience and guidance from diabetes experts suggest that, for many patients, Mounjaro becomes a long‑term therapy similar to metformin or insulin when the goal is ongoing glycemic control.
For weight management: evidence from trials of tirzepatide used specifically for obesity (notably the SURMOUNT program) shows large weight losses while people are on therapy, but substantial weight regain tends to occur after stopping the drug. That means if your goal is sustained weight loss, you and your clinician will need to discuss whether continued treatment or a structured maintenance plan is the best path.
- Practical example: someone with newly diagnosed type 2 diabetes may start Mounjaro and keep it long-term because stopping leads to rising A1C and return of symptoms.
- Contrast: a person using tirzepatide for significant weight loss may hit their target after many months and then choose a trial off the drug — but many patients and clinicians see weight creep back without ongoing strategies or medication.
Experts emphasize individualized decision-making. Endocrinologists will weigh metabolic benefits against side effects, cost, access, and patient preference. Ask yourself: do I need ongoing glucose control, or is my aim temporary weight reduction? Is the medication tolerable and affordable long-term?
Monitoring and reassessment: whether short- or long-term, periodic check-ins are essential — review weight trends, A1C or glucose metrics, side effects, blood pressure, and how the medication fits into your daily life. If symptoms or labs change, your provider may alter dose, pause therapy, or add other treatments.
Boxed Warning: Risk of Thyroid Cancer
What should make you pause and ask questions before starting Mounjaro? The FDA requires a boxed warning about a potential risk of thyroid C‑cell tumors, based on findings in rodent studies — and that forms a very important part of prescribing decisions.
What the warning says: in animal studies tirzepatide and some drugs in the GLP‑1 receptor agonist class caused thyroid C‑cell tumors (including medullary thyroid carcinoma, or MTC). It’s not known if this applies to humans, but because the risk can’t be ruled out, the label includes a boxed warning and specific contraindications.
- Who should NOT take Mounjaro: people with a personal or family history of medullary thyroid carcinoma (MTC) or those with Multiple Endocrine Neoplasia syndrome type 2 (MEN2).
- What to watch for: seek evaluation for persistent neck swelling, a lump in the neck, difficulty swallowing, or unexplained voice changes — these can be warning signs clinicians monitor for.
- Clinical context: experts point out that rodent thyroid physiology differs from humans, so the animal findings don’t translate directly, but the boxed warning remains a cautious, safety‑driven policy.
In practice, clinicians take a pragmatic approach: they screen for personal or family history of MTC/MEN2, counsel patients on signs to watch for, and document informed discussion of risks and benefits. If you or a family member have a relevant history, your provider will typically choose a different treatment.
Finally, remember there are other safety considerations beyond thyroid cancer — for example, nausea, gallbladder events, and rare reports of pancreatitis — and these are part of the shared decision when starting therapy.
Dosing for Weight Loss
Curious how dosing works when tirzepatide is used for weight loss? Let’s walk through a typical approach, why titration matters, and what the evidence shows.
Typical titration schedule used in obesity trials and clinical practice: clinicians generally follow a gradual, weekly administration plan to improve tolerability. Below is a common stepwise schedule modeled after large clinical trials (for tirzepatide used for weight management):
- Weeks 1–4: 2.5 mg once weekly (starter dose to reduce gastrointestinal side effects).
- Weeks 5–8: increase to 5 mg once weekly if tolerated.
- Weeks 9–12: increase to 7.5 mg once weekly as needed for efficacy.
- Subsequent increases: some people advance to 10 mg, then 12.5 mg, and up to 15 mg once weekly — each step usually spaced about 4 weeks apart and based on tolerability and weight-loss response.
In the SURMOUNT trials, fixed doses like 5, 10, and 15 mg produced progressively greater average weight loss across arms, with the highest dose producing the largest reductions in body weight over 72 weeks. That gives a clear signal: more drug often equals more benefit, but also more side effects for some people.
Important practical points:
- Start low, go slow: the initial low dose reduces nausea, which is the most common barrier to continuing therapy.
- Individualize the target dose: your clinician will balance how much weight loss you want with side effects, cost, injection comfort, and medical history.
- Off‑label considerations: as of mid‑2024, Mounjaro is FDA‑approved for type 2 diabetes; tirzepatide branded for weight management (Zepbound) has separate labeling. Some clinicians use Mounjaro off‑label for weight loss and follow the tirzepatide weight‑management titration, but you should discuss legal, insurance, and safety implications with your provider.
Realistic expectations and maintenance: people in the obesity trials lost substantial weight over many months, but weight regain after stopping therapy is common. That means dosing for weight loss is often seen as a bridge to long‑term lifestyle changes and potentially ongoing medication for maintenance if needed.
Ask yourself: How much weight loss do I hope to achieve? How important is avoiding side effects? Are you prepared for long‑term planning if the medication is successful? Bringing these questions to the clinic will help shape a dosing strategy that matches your goals.
What Is the Recommended Mounjaro Dose for Weight Loss?
Curious about what dose of Mounjaro (tirzepatide) people use when their goal is weight loss? You’re not alone — patients and clinicians often ask the same thing because Mounjaro was developed for type 2 diabetes but has powerful effects on weight that many want to harness safely.
What clinicians commonly use: In practice, many prescribers follow a gradual weekly titration that balances effectiveness with tolerability. A typical regimen looks like this:
- Start: 2.5 mg once weekly for 4 weeks (this is a low introductory dose to help your body adjust and reduce nausea).
- Step-up: Increase to 5 mg once weekly for at least 4 weeks.
- Further increases: If tolerated and if more weight loss is desired, move to 10 mg once weekly, and in some cases to 15 mg once weekly as the highest commonly used dose.
Why we titrate slowly: Gastrointestinal side effects (nausea, vomiting, diarrhea, constipation) are the most common reason to move slowly. A gradual increase gives many people a chance to adapt and reduces the need to stop therapy.
What the evidence shows: Clinical development programs for tirzepatide (the SURMOUNT studies and other trials) demonstrated substantial, often double-digit percentage weight loss in many participants when higher maintenance doses were used. These trials also showed a dose–response pattern: higher doses tend to produce more weight loss but also more side effects. That’s why individualized titration matters.
Practical tips:
- Work with your prescriber to set realistic goals and decide on a target maintenance dose — not everyone needs or tolerates the highest dose.
- Expect the first month to be about tolerability rather than maximal weight loss.
- If side effects are troublesome, holding or stepping back to the prior tolerated dose for a few weeks often helps.
- Combine medication with a nutrition and activity plan — the medication is a tool, not a standalone magic fix.
Safety first: Always discuss medical history (pancreatitis, gallbladder disease, pregnancy plans, or current insulin/sulfonylurea use) with your clinician, because dosing and monitoring decisions change with those factors.
Switching and Equivalency Between Drugs
Thinking about changing from one injectable weight-loss drug to another? It’s a common question, and you’ll want clarity about equivalency, safety, and what the transition feels like.
Are doses directly equivalent? No — we can’t convert semaglutide (Wegovy) milligrams into tirzepatide (Mounjaro) milligrams the way you might convert units of insulin. They are different molecules with different mechanisms: semaglutide is a GLP‑1 receptor agonist, while tirzepatide is a dual GIP/GLP‑1 receptor agonist. That means their potency and side-effect profiles are distinct, so equivalence is clinical rather than mathematical.
Clinical approach to switching:
- Assess the reason for switching: plateau in weight loss, intolerable side effects, cost/insurance issues, or desire for greater efficacy.
- Expect a new titration: most clinicians start tirzepatide at a low introductory dose (commonly 2.5 mg weekly) and titrate up regardless of prior semaglutide dose, to minimize nausea and GI upset.
- Consider timing: because both drugs are weekly injections, some clinicians wait one dosing interval after the last dose of the prior drug before starting the new one; others start the new agent at the next scheduled dose. This decision depends on tolerability, diabetes control, and clinician judgment.
- Watch for duplicated effects: if you’re on other glucose-lowering agents (insulin, sulfonylureas), doses may need adjustment to avoid hypoglycemia during the switch.
What the evidence and expert opinion say: While head-to-head randomized trials comparing tirzepatide and semaglutide for weight loss are limited, aggregated data and clinical experience suggest tirzepatide often produces greater average weight loss at comparable treatment durations. That potential benefit must be weighed against side effects and cost/coverage considerations.
Monitoring during transition: Monitor symptoms (GI side effects), weight trajectory, blood glucose if diabetic, and general labs as guided by your provider. Rapid weight loss can increase the risk of gallstones and changes in electrolytes or liver tests, so stay in communication with your care team.
Can You Switch From Wegovy to Mounjaro?
Short answer: Yes, many people can switch from Wegovy (semaglutide) to Mounjaro (tirzepatide), but it should be done thoughtfully and under medical supervision. Let’s walk through how that often looks in real life.
Why someone might switch:
- Plateau in weight loss on Wegovy despite good adherence.
- Preference to try a different mechanism that may yield more weight loss.
- Side effects on Wegovy that might be better with a different agent (or vice versa).
- Insurance coverage changes or medication availability.
A typical switching plan (example): Imagine Sarah, who has been on Wegovy 2.4 mg weekly for 9 months and saw an early large loss but recently plateaued. Her clinician might:
- Review her history and labs, confirm no contraindications (pregnancy, pancreatitis history, etc.).
- Discuss expectations — tirzepatide may produce further weight loss, but nausea can increase at higher doses.
- Stop Wegovy at the next scheduled dose or after an appropriate interval agreed with the clinician.
- Start tirzepatide at 2.5 mg once weekly and plan stepwise increases every 4 weeks as tolerated (5 mg → 10 mg → possibly 15 mg), with close follow-up for side effects and weight response.
- Adjust any diabetes medicines to lower hypoglycemia risk if needed.
What to expect emotionally and physically: Switching can be exciting — because there’s potential for new progress — and a little anxious, because the body needs to re-adapt. You may experience a brief period of increased GI side effects during the first several weeks of tirzepatide titration. Many people report renewed momentum in weight loss after a switch, while others see only modest change.
Questions to ask your clinician before switching:
- Why do you recommend switching now?
- What starting dose and titration schedule will we use?
- How will my other medications (especially diabetes meds) be adjusted?
- What side effects should prompt me to call or stop the medication?
- How will we monitor progress and safety (labs, follow-up frequency)?
Final thought: Weigh the potential for greater weight loss against the possibility of different side effects and insurance hurdles. With careful planning, many people successfully switch from Wegovy to Mounjaro and continue toward their weight and health goals while staying safe and supported.
Can You Go Straight From Ozempic to Mounjaro?
Have you ever wondered whether you can switch from Ozempic (semaglutide) to Mounjaro (tirzepatide) in one step? The short answer is: often yes, but it’s not as simple as swapping pens. Both drugs are weekly injectables used for type 2 diabetes (and sometimes for weight management), but they work slightly differently — Ozempic is a GLP‑1 receptor agonist, while Mounjaro is a dual GIP/GLP‑1 receptor agonist — and that changes how we approach switching.
Key practical point: many clinicians start Mounjaro at the recommended initiation dose even if a patient has been on a GLP‑1 such as semaglutide, because starting low helps avoid gastrointestinal side effects and allows safe titration.
- Why we usually don’t go “full dose” immediately: tirzepatide can cause stronger nausea, vomiting, or diarrhea in people new to it or after a high GLP‑1 exposure. Starting at the labeled initiation dose (commonly 2.5 mg weekly for 4 weeks) then increasing gradually is the standard to improve tolerability.
- Timing and overlap: there’s generally no long drug washout required because both are weekly agents with similar half-lives, but follow manufacturer guidance and your prescriber’s plan. Your provider will consider your current dose, blood sugar control, other diabetes medicines (insulin or sulfonylureas raise hypoglycemia risk), and side‑effect history.
- Medical caution points: if you’ve had pancreatitis, a history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 (conditions flagged in GLP‑1 safety warnings), pregnancy/planning pregnancy, or severe GI disease, switching requires a thoughtful risk–benefit conversation.
Think of the switch like changing lanes on a busy road: you can do it, but you slow down, signal, and check your mirrors. Many endocrinologists will say, “Let’s reduce the chance of turbulence — we’ll start low and go slow.” Real‑world practice and trial protocols (for example, the SURPASS program that evaluated tirzepatide) reinforce this cautious, stepwise approach.
Have you discussed switching with your provider yet? If not, bring your most recent glucose logs and a list of current medications — that information helps tailor the transition plan so you and your care team can minimize side effects and maintain glycemic safety.
How Much Mounjaro Is Equivalent to 1 Mg of Ozempic?
Wouldn’t it be convenient if we could convert milligrams across drugs like units on a recipe card? Unfortunately, there’s no reliable mg‑to‑mg equivalence between Ozempic (semaglutide) and Mounjaro (tirzepatide). They are different molecules with different receptor targets and potencies, so a dose of one does not map directly to a dose of the other.
What the research tells us: head‑to‑head trials such as SURPASS‑2 compared various doses of tirzepatide (5 mg, 10 mg, 15 mg) to semaglutide 1 mg and found that tirzepatide produced greater reductions in HbA1c and body weight than semaglutide 1 mg. That shows relative clinical effect — not an exact milligram conversion.
- Clinical approach instead of math: clinicians compare effects (glucose lowering, weight loss, side effects) rather than attempt a numeric conversion. For example, if you’re on semaglutide 1 mg weekly for diabetes and your provider wants to move to tirzepatide, they may recommend starting tirzepatide at the labeled initiation dose (e.g., 2.5 mg weekly for 4 weeks) then titrating to 5 mg and upward as tolerated and needed — not an immediate jump to a high tirzepatide dose simply to “match” semaglutide 1 mg.
- Individual goals matter: if your priority is tighter blood sugar control, your provider will titrate until your HbA1c target is met; if weight loss is the main goal, they may push titration further (with careful monitoring). The final tirzepatide dose that achieves the same clinical outcome as semaglutide 1 mg will vary between people.
- Safety-first example: someone on insulin plus semaglutide 1 mg who switches to tirzepatide may need insulin dose adjustments to avoid hypoglycemia. A stepwise titration minimizes the risk of severe GI intolerance which might otherwise lead to stopping therapy.
So, rather than asking “How many mg of tirzepatide equals 1 mg of semaglutide?”, a more useful question is “What tirzepatide dose will give you the same clinical benefits with acceptable side effects?” That’s a conversation to have with your clinician, informed by your glucose data, weight goals, co‑medications, and tolerance history. We recommend sharing your experience with Ozempic (how you felt, side effects, and how well your sugars were controlled) so your provider can design a personalized titration plan.
Missed Dose, Overdose, and Scheduling Changes
Have you ever missed a weekly injection and panicked about what to do next? Or wondered what happens if you accidentally take too much? We’ve all been there — life gets busy. Here’s practical, clinician‑oriented guidance to help you navigate missed doses, potential overdoses, and how to change your injection schedule without destabilizing your treatment.
Missed Dose: What to do
General rule: for weekly GLP‑1/GIP therapies, the manufacturer guidance usually allows a short window after the missed dose to take it; beyond that window you skip the missed dose and resume your regular schedule. The precise window differs by product, so check the specific prescribing information and talk to your provider or pharmacist.
- Typical practical steps: if you remember within a few days (commonly 3–5 days depending on the medicine), take the missed dose as soon as you remember and then continue your usual weekly schedule. If the missed dose would require you to take two doses too close together to get back on schedule, skip the missed dose and take the next dose on your normal day.
- Why the window matters: exceeding the recommended interval can reduce drug levels and clinical effect; taking doses too close together can increase side effects. Asking your clinic or pharmacist for the exact timing rule for your prescription is an easy way to avoid mistakes.
Overdose: Signs and immediate actions
Symptoms to watch for: severe nausea, vomiting, persistent abdominal pain (possible pancreatitis), severe dehydration, or profound weakness. If you’re taking other diabetes medicines that can cause low blood sugar (insulin or sulfonylureas), an overdose — or rapid increased effect — could lead to hypoglycemia (dizziness, sweating, confusion, fainting).
- Immediate steps: if you suspect a large overdose or if severe symptoms occur, seek emergency care. For mild excess dosing with tolerable symptoms, contact your prescribing clinician or poison control for advice; treatment is generally supportive (fluids, antiemetics) and monitoring.
- Prevention tip: use pillbox labels, weekly calendars, reminder apps, or an injection tracker to prevent accidental double dosing.
Changing your weekly injection day
Want to move your weekly injection day from Monday to Thursday? You can — with a little planning.
- Short move (within the allowed missed‑dose window): if your new day falls within the same allowable missed‑dose window (for example, within 3–5 days), you can take the dose on the new day and continue weekly from there.
- Longer shift: if the gap would exceed the manufacturer’s missed‑dose window, it’s safer to take the dose on the new day only if you’ve confirmed with your prescriber; otherwise, take the next dose on your usual day and then gradually move the scheduled day by taking doses a day earlier or later within allowed windows until you reach the preferred day.
- Consistency helps: once you choose a new regular day, try to stick to it — consistent timing supports steady drug levels and predictable effects.
Finally, remember this: whenever you make changes — missed doses, timing switches, or if an overdose occurs — loop in your healthcare team promptly, especially if you’re on insulin or sulfonylureas. We want to keep you safe, comfortable, and on track with your goals; a short phone call can prevent bigger problems later. What’s your experience been with weekly injections — do reminders help you stay consistent, or do you prefer linking the shot to a weekly habit (like trash day or a Sunday ritual)?
What Should I Do If I Miss a Dose?
Have you ever gone to take a weekly injection and realized you forgot? It happens to all of us — the key is how you handle it. With Mounjaro (tirzepatide) the general, manufacturer-recommended approach is practical and safety-focused: if you remember within a few days, take the missed dose as soon as you remember; if more time has passed, skip it and return to your regular weekly schedule. This helps you avoid stacking doses and reduces the chance of intensified side effects like nausea or dizziness.
Why that cautious approach? Clinical trials such as the SURPASS and SURMOUNT programs showed that common adverse effects are dose-related and often gastrointestinal. Experts — endocrinologists and diabetes educators — routinely emphasize avoiding “double-dosing” because taking two doses too close together can spike side effects without improving benefit.
- Practical example: If your usual day is Friday and you realize on Monday you missed Friday’s dose, most guidance allows you to take the missed dose and then continue each week on Monday. If you realize two weeks later, skip the missed one and take your next scheduled dose that week.
- What not to do: Don’t take two doses on the same day or try to “catch up” by taking an extra injection — that increases side effects and isn’t supported by evidence.
- When to call your clinician: If you’re on insulin or a sulfonylurea, if you have severe vomiting/diarrhea, or if you’re unsure how long it’s been, reach out so your provider can advise on dosing and blood glucose monitoring.
We all juggle busy lives — setting a phone reminder or pairing your shot day with a weekly habit (laundry day, Sunday coffee) can prevent missed doses. If you’re ever in doubt, check the product information or ask your pharmacist; they’re used to guiding people through these exact scenarios.
What Should Be Done in Case of Overdose?
What if you — or someone else — accidentally takes too much? First, take a breath — overdoses of Mounjaro are usually managed supportively rather than with a specific antidote. That said, you should act quickly and calmly.
Immediate steps:
- Contact emergency services or your local poison control center right away if there are severe symptoms (very low blood sugar, fainting, severe vomiting, breathing difficulties, or altered consciousness).
- Monitor blood glucose closely, especially if you use insulin or sulfonylureas, because combining those drugs with a GLP-1/GIP agonist can increase the risk of hypoglycemia.
- Keep hydrated and seek medical evaluation — treatment is primarily supportive: IV fluids for dehydration, antiemetics for severe nausea and vomiting, and glucose or glucagon for significant hypoglycemia.
Why take this seriously? Trial data and post-marketing experience show that the most common severe issues with higher-than-prescribed exposure are pronounced gastrointestinal symptoms and hypoglycemia when other glucose-lowering meds are involved. Emergency clinicians will stabilize vital signs, manage blood sugar, and treat symptoms while monitoring for complications.
As a practical note, keep the medication box and pen with you if you go to urgent care or the ER — that helps clinicians confirm dose and timing. And afterwards, talk with your prescriber about why the overdose occurred and whether any changes (education, different delivery device, or once-weekly reminders) might reduce future risk.
What If I Want to Change the Day I Take Mounjaro?
Thinking of switching your injection day to better fit your routine? That’s a smart move for adherence. The good news: you can change your weekly day, but you should do it thoughtfully so you don’t shorten the interval between doses or accidentally double-dose.
How to switch safely:
- If it has been only a short time since your last dose, it’s usually safest to wait until your next scheduled injection and then begin taking it on the new chosen day each week.
- If a few days have passed and your clinician or the medication guide indicates it’s okay to take the missed/next dose early, you can take it on the new day and continue weekly from there. The key principle is to avoid administering more than one full dose in a single 7‑day period.
Picture it like moving a recurring appointment: we don’t want to schedule two appointments back-to-back; instead we shift the pattern forward and keep the rhythm consistent. Endocrinologists and diabetes educators often recommend picking a steady weekly anchor — for example, “I’ll always take it every Tuesday after my morning shower” — because routines stick.
Before you make the switch, ask yourself:
- Are you taking insulin or other medications that might need temporary adjustment?
- Is there a travel or life event that means your new day will be more sustainable long-term?
If the answer suggests complexity, run the plan by your clinician or pharmacist. They can advise on whether any short-term glucose monitoring or medication adjustments are needed during the transition. Changing the day can be simple, but doing it with a little planning keeps your treatment safe and effective — and helps you stay on track for the long run.
Side Effects and Interactions
Have you ever started a new medication and wondered which side effects are “normal” and which ones need a phone call to your provider? With Mounjaro (tirzepatide), that question is common—and wise to ask. In clinical trials and real-world use, the most frequent side effects are related to the digestive system: nausea, vomiting, diarrhea, constipation and decreased appetite. These tend to occur early, are often mild-to-moderate, and usually improve as your body adapts.
Beyond the tummy troubles, there are other important considerations. Injection-site reactions can happen, and some people report feeling fatigued or having mild dizziness. There are also more serious, but less common, risks to be aware of: several cases of pancreatitis have been reported with incretin-based therapies (the drug class that includes tirzepatide), and there’s a theoretical risk for gallbladder disease as rapid weight loss can predispose to gallstones.
One unique safety note: animal studies showed an increased incidence of thyroid C‑cell tumors with tirzepatide-like drugs in rodents. While human relevance is unclear, manufacturers recommend that people with a personal or family history of medullary thyroid carcinoma or MEN2 avoid the drug. Always mention those family history details when you talk with your clinician.
How likely are these side effects? In the SURPASS clinical program, tirzepatide produced meaningful reductions in HbA1c and body weight versus comparators, but gastrointestinal symptoms were the most common adverse events and were the primary reason some participants stopped treatment. Experts often compare starting tirzepatide to learning a new exercise routine: at first your body protests, but with gradual increases and the right adjustments (and support), many people tolerate it well and reap the benefits.
Finally, interactions: tirzepatide itself is not a major CYP enzyme substrate, so it’s less likely to interact through liver enzyme pathways that change drug levels. However, because it slows gastric emptying, it can alter the absorption of oral medications—especially those that need consistent absorption (such as certain birth control pills, antibiotics, or drugs with a narrow therapeutic index). For those on medications like warfarin, anticonvulsants, or thyroid hormone, we watch closely for changes in effect when starting or stopping Mounjaro.
Bottom line: the common side effects are largely gastrointestinal and transient, but there are important, rarer risks and practical interactions to discuss with your clinician so we can monitor and adjust safely.
Does Mounjaro Interact with My Other Drugs?
Worried about mixing Mounjaro with your other prescriptions? That’s a great question—many of us are taking several meds, and understanding interactions keeps things safe and predictable. The main interaction concern with tirzepatide is not so much a drug‑metabolizing enzyme problem, but a pharmacokinetic and pharmacodynamic one.
First, the pharmacokinetic angle: tirzepatide can slow gastric emptying. In practice, that means pills you take by mouth may be absorbed more slowly. For most medications this won’t make a big difference, but for drugs where timing and steady levels matter (for example, certain oral contraceptives, some antibiotics, or drugs with narrow therapeutic windows), this delayed absorption could change effectiveness. Clinicians often advise monitoring—if you notice reduced effect of a medication after starting Mounjaro, tell your provider so they can consider timing adjustments or alternative formulations.
Second, the pharmacodynamic angle: tirzepatide lowers blood sugar and reduces appetite and weight. If you’re already on other glucose-lowering drugs—especially insulin or sulfonylureas—you’re at higher risk for hypoglycemia. That interaction is clinically meaningful and common enough that standard practice is to plan for dose changes when initiating Mounjaro (more on that below).
Other practical interactions to watch for:
- Anticoagulants (e.g., warfarin): Any change in oral absorption, appetite, or weight might affect INR variability—monitor INR more frequently when starting or stopping tirzepatide.
- Oral contraceptives: If you rely on pills and experience persistent vomiting or diarrhea, effectiveness could be reduced—consider backup contraception until GI symptoms settle.
- Drugs requiring strict timing: Meds taken on an empty stomach or with specific timing may need re-evaluation if gastric emptying changes your symptomatic routine.
Experts recommend a few simple steps to manage interactions: tell every member of your care team what you’re starting, keep a list of all your meds and supplements, monitor for new or changing symptoms, and plan follow-up bloodwork or drug‑level checks when appropriate. In many cases, careful monitoring rather than stopping a needed medication is the right approach.
Will My Insulin Dose Change When I Start Receiving Mounjaro?
Short answer: quite possibly—yes. Let’s walk through why and how.
Mounjaro significantly improves glycemic control in many people with type 2 diabetes, meaning your blood sugar may drop faster or further than before. If you keep the same insulin dose, that increases your risk for low blood sugar. Because of that, endocrinologists and primary care providers commonly adjust insulin or sulfonylurea doses when initiating tirzepatide.
How those adjustments look in practice depends on what kind of insulin regimen you’re on:
- Basal insulin only: Many clinicians reduce basal insulin by about 10–20% at or shortly after starting Mounjaro, then titrate back up or down based on home glucose readings. The goal is to avoid hypoglycemia while still preserving the long‑term glycemic benefit.
- Basal–bolus (mealtime) insulin: Patients often keep a conservative approach with basal insulin reductions first and reassess prandial (mealtime) doses. Because tirzepatide reduces postprandial glucose excursions, you may find you need less bolus insulin over time—sometimes eliminating mealtime injections if glycemic targets can be kept with fewer shots.
- Insulin with sulfonylurea or meglitinide: Because these drugs raise hypoglycemia risk in combination, providers frequently lower or stop the sulfonylurea when starting tirzepatide.
Clinical trial context helps: in the SURPASS studies, tirzepatide produced substantial HbA1c reductions versus comparators, and among patients using insulin, hypoglycemia rates were impacted by concurrent sulfonylurea or insulin use. That’s why trial protocols and real‑world guidance emphasize monitoring and often preemptive insulin dose reduction.
What should you expect during the transition?
- Increased glucose monitoring: Check more often in the first 2–8 weeks—fasting, pre‑meal, and occasional post‑prandial checks help your clinician fine‑tune doses.
- Rule of thumb reductions: Some clinicians use an initial basal insulin cut of 10–20% or reduce mealtime insulin if you’re experiencing low sugars. These are starting points, not rules—your response will guide ongoing changes.
- Watch for symptoms: Learn hypoglycemia signs (sweating, shakiness, confusion) and carry quick sugar sources. Likewise, report severe nausea, vomiting, or dehydration quickly, because these can affect insulin needs and kidney function.
Let me share a common scenario: a patient I followed started tirzepatide while on 40 units of basal insulin nightly. Their provider advised a 15% drop to 34 units and increased glucose checks for two weeks. Within a month their fasting sugars were lower than expected and they reported fewer snacks; the basal dose was reduced further to 30 units and mealtime insulin—previously 6–8 units—was nearly stopped. This kind of stepwise reduction is typical and often rewarding: better control, less insulin, and meaningful weight loss. But it takes teamwork and monitoring.
Final takeaways: plan for possible insulin dose changes, increase glucose monitoring when starting Mounjaro, and stay in close contact with your care team. Weigh the benefits—improved A1c and weight loss—against the temporary adjustments and vigilance required to prevent hypoglycemia. Your clinician will tailor the approach to your regimen, lifestyle, and preferences so we can make the transition both safe and effective.
Do I Need to Change My Mounjaro Dosage If I Take Birth Control Pills?
Worried that your weekly Mounjaro shot might interfere with your birth control? That’s a smart question and one many people ask when starting a new medication.
The short answer: for most people, you won’t need to change the dose of your oral contraceptive when you start Mounjaro (tirzepatide). Clinical data and the drug’s prescribing information do not indicate a direct pharmacokinetic interaction that reduces the effectiveness of combined hormonal contraceptives.
That said, there are important practical details to keep in mind. Mounjaro commonly causes gastrointestinal side effects—nausea, vomiting, and diarrhea—especially when you’re titrating up. If you experience significant vomiting or prolonged diarrhea after your injection, your body may not absorb an oral contraceptive correctly that day or over several days. In that scenario, typical contraceptive counseling applies: treat the missed/affected contraception days as higher-risk and use backup contraception (e.g., condoms) until you’ve taken active pills reliably for 7 days, or follow the specific guidance in your pill’s prescribing leaflet.
Think about how you manage other short-term stomach bugs: if you vomit soon after taking a pill, you and your clinician would usually recommend a backup method. Mounjaro-related nausea is similar in that sense. If you’re on a long-acting method (IUD, implant, injection), those aren’t affected by absorption and you likely don’t need to change anything.
Other considerations: rapid, substantial weight loss can sometimes change hormone levels and bleeding patterns, but it’s not a reason to stop or change your birth control proactively. If you’re trying to conceive, or if there’s any chance you might be pregnant, discuss stopping Mounjaro with your prescriber because tirzepatide is not recommended during pregnancy.
Practical example: Emily starts Mounjaro and experiences nausea during weeks 1–4. She continues her combined oral contraceptive but uses condoms for the first week after a couple of vomiting episodes until she’s confident she’s reliably absorbing her pills. Her clinician reassures her this is a common, temporary precaution and helps plan symptom management to minimize missed-dose risk.
If you’re unsure, bring this up with your prescribing clinician or pharmacist so you get individualized advice based on your contraceptive method and how severe your GI symptoms are.
What If I Can’t Tolerate My New Dose?
Starting a new dose that makes you feel awful can be discouraging—what do you do when the side effects seem worse than the benefit? We’ve all been there with medications. Let’s walk through practical, evidence-informed steps you and your clinician can consider.
1) Slow down the titration. One of the best ways to improve tolerability is to pause on dose escalation or step back to the previous dose for a few weeks. For weekly injectables like Mounjaro, many clinicians recommend staying at a lower dose longer and only increasing when GI symptoms have settled. Trials with GLP-1 therapies have shown that slower titration can reduce nausea and vomiting.
2) Symptom management strategies. Small behavioral tweaks often help: eat smaller, more frequent meals; avoid very fatty or spicy foods around the time you expect nausea; stay well hydrated; and use ginger or bland snacks. For persistent nausea, prescribers sometimes recommend short courses of antiemetics (for example, ondansetron or meclizine) while symptoms are worst. Always check with your clinician before adding another drug.
3) Injection technique and timing. Rotate injection sites, ensure the medication is at room temperature when injected, and pick a consistent day/time each week. For some people, timing the injection on a day when they can take it easy or have easy-to-digest food can make initial side effects more manageable.
4) Consider dose reduction or discontinuation. If symptoms are intolerable despite slower titration and supportive measures, you and your clinician may choose to reduce the dose back to a level you tolerated or stop Mounjaro and switch to an alternative treatment. There are other classes of diabetes and weight-management medications with different side effect profiles that may be better suited to you.
5) Watch for red flags. If you develop severe abdominal pain, persistent vomiting, signs of pancreatitis (severe upper abdominal pain radiating to the back), or sudden vision changes (particularly with rapid glucose lowering), seek medical evaluation promptly. GLP-1/GIP therapies have been associated with rare but serious adverse events that require immediate attention.
Example narrative: When Raj moved from 2.5 mg to 5 mg he had increased nausea and skipped meals—so his clinician suggested staying at 2.5 mg for an extra 4 weeks and prescribed a short antiemetic for nighttime nausea. Once his stomach settled, he worked up to 5 mg slowly and tolerated it fine. That pause kept him on therapy rather than quitting entirely.
In short, intolerable side effects don’t have to mean the end of therapy. We can often manage them by slowing titration, treating symptoms, and making small lifestyle or technique tweaks—always in partnership with your prescriber.
Prescribing and Professional Resources
Are you a clinician or someone who wants the clinical playbook? Here are practical prescribing points, monitoring tips, and where clinicians typically look for deeper guidance.
- Typical Mounjaro dosing pathway: start 2.5 mg once weekly for 4 weeks (to improve tolerability), then increase to 5 mg once weekly. If additional glycemic control is needed, titrate upward in 4-week intervals to 7.5 mg, 10 mg, and up to 15 mg once weekly, based on response and tolerability. Many prescribers extend each step beyond 4 weeks when GI tolerance is a concern.
- Baseline evaluation: review history of pancreatitis, gallbladder disease, med list (especially other hypoglycemics), pregnancy potential, and a baseline HbA1c and renal function. Counsel about potential GI side effects and signs that warrant urgent care.
- Monitoring: follow HbA1c and glucose per diabetes guidelines, monitor weight, and watch for GI adverse events. If there’s rapid improvement in glycemic control, be mindful of retinopathy risk in those with existing diabetic retinopathy and coordinate with ophthalmology when appropriate.
- Drug interactions and contraception: no clear pharmacokinetic interaction with combined oral contraceptives has been identified, but counsel patients that vomiting/diarrhea can reduce oral contraceptive absorption and backup contraception may be needed temporarily.
- When to refer: complex cases—history of severe GI disease, recurrent pancreatitis, pregnancy planning, or uncertain diagnosis of type 1 diabetes—warrant endocrinology consultation.
- Key evidence and guidance sources clinicians consult: FDA prescribing information for tirzepatide, SURPASS efficacy and safety trials for type 2 diabetes, SURMOUNT trials for weight outcomes, and professional society guidance (ADA, AACE, Endocrine Society) for glucose-lowering strategies. These documents address dosing schedules, safety signals, and population-specific considerations.
- Practical clinic tips: provide written titration schedules, explain injection technique, ask patients to keep a brief side-effect diary during dose changes, and schedule follow-ups early in the titration period so you can troubleshoot quickly.
If you’d like, I can create a simple printable dosing checklist or a patient handout that explains titration steps, symptom management tips, and when to call the clinic—would that help you or your patients?
Ready to Prescribe Mounjaro?
Are you wondering whether Mounjaro (tirzepatide) could be the right next step for a patient with type 2 diabetes? Picture a patient you’ve treated for years — modestly elevated A1c despite metformin and lifestyle changes, carrying extra weight, frustrated by limited options. In recent years, tirzepatide has shown striking benefits in the SURPASS program: clinicians report meaningful A1c declines and substantial weight loss compared with many other therapies, which is why many of us are asking, “Should I offer this?”
Key considerations before you prescribe:
- Indication: For adults with type 2 diabetes as an adjunct to diet and exercise — not for type 1 diabetes or diabetic ketoacidosis.
- Benefits seen in trials: Greater glycemic and weight reductions versus several comparators (including semaglutide 1 mg in SURPASS‑2), which many clinicians cite as practice-changing.
- Safety signals: Expect GI side effects (nausea, vomiting, diarrhea) most commonly during titration; rare but important concerns include pancreatitis and potential thyroid C‑cell effects.
- Practical barriers: Injection training, prior authorization paperwork, and out‑of‑pocket cost can affect uptake — plan ahead to support adherence.
So before you write the script, ask: what are my patient’s goals, how tolerant are they of GI symptoms, and can we commit to the stepwise titration that maximizes tolerability and efficacy?
How to Prescribe Mounjaro
Let’s walk through a practical, clinician-friendly checklist you can use in the clinic — the sort of workflow that turns a potentially daunting prescription into a straightforward plan you and your patient can follow together.
- Baseline assessment: Confirm type 2 diabetes diagnosis, obtain A1c, BMP (for renal baseline), liver tests if indicated, and a focused history for pancreatitis, gallbladder disease, and family history of medullary thyroid carcinoma or MEN2.
- Medication reconciliation: Identify sulfonylureas or insulin that may require dose reduction to avoid hypoglycemia when starting Mounjaro; consider reducing these agents preemptively if the patient has tight control or is symptomatic.
- Informed consent and counseling: Discuss expected benefits, common side effects (especially during dose escalation), injection technique, and the likely need for prior authorization — be transparent about cost and assistance programs.
- Write the prescription: Include start dose, titration schedule (see dosing section), refill strategy, and dosing frequency (once weekly). Example prescription text you can adapt: “Tirzepatide (Mounjaro) 2.5 mg subcutaneous pen — inject once weekly. Increase to 5 mg after 4 weeks, then escalate by one step every 4 weeks as tolerated to a maximum of 15 mg weekly for glycemic control. Provide education on injection and side‑effect management. Reduce sulfonylurea/insulin per plan.”
- Monitoring plan: Schedule follow-up at ~4 weeks after initiation to assess tolerability, then every 4–12 weeks during titration; check A1c about 3 months after starting or changing dose, monitor weight, and reassess other glucose‑lowering meds.
- Documentation for payors: Be prepared to document prior treatment failures/intolerance and A1c goals, and attach education notes and a titration plan to speed authorization.
When you talk through these steps with the patient — acknowledging fears about injections and cost, sharing realistic timelines for benefit, and offering your support through titration — uptake and persistence tend to improve.
How to Dose Mounjaro
Want a clear, usable titration chart you can discuss with patients in the exam room? Here’s a straightforward, evidence‑informed dosing approach based on the FDA labeling and common clinical practice — designed to minimize GI upset while reaching effective doses.
- Starting dose: 2.5 mg subcutaneously once weekly for 4 weeks. This is a tolerability starter dose and provides no long‑term glycemic effect by itself; it reduces GI side effects when escalating.
- Escalation steps (typical):
- After 4 weeks at 2.5 mg → increase to 5 mg weekly.
- If additional glycemic control is needed and the dose is well tolerated, increase every 4 weeks as follows: 5 mg → 7.5 mg → 10 mg → 12.5 mg → 15 mg weekly.
- Maximum dose: 15 mg once weekly.
- When to escalate: Only increase after at least 4 weeks at the current dose and when GI symptoms are tolerable. If side effects are troublesome, hold escalation or step back until symptoms improve.
- Clinical examples:
- Patient A (A1c 8.5%, sensitive to nausea): Start 2.5 mg x4 weeks → 5 mg x4–8 weeks; reassess A1c and side effects before further escalation.
- Patient B (A1c 9.8%, willing to accept side effects to lower A1c quickly): Start 2.5 mg x4 weeks → 5 mg x4 weeks → 10 mg if tolerated, with closer follow‑up and anticipatory guidance for GI symptoms.
- Administration tips: Give subcutaneously once weekly on the same day each week (any time of day, with or without food). Rotate injection sites (abdomen, thigh, upper arm) and counsel on needle disposal and storage (keep pens refrigerated unopened and protect from freezing).
- Mitigating side effects: Advise smaller, more frequent meals, avoid fatty or large meals when nauseated, keep hydrated, and consider antiemetics for severe nausea after evaluating causes. Titration pace is your main tool to reduce GI intolerance.
- Drug interactions and adjustments: Reduce insulin/sulfonylurea doses when starting or increasing tirzepatide to lower hypoglycemia risk; monitor closely. No routine renal dosing adjustment is generally required, but use caution in severe renal impairment and in patients with hepatic impairment.
- Monitoring: Check weight and symptoms at each visit; A1c at roughly 3 months after initiation or dose change; remain vigilant for signs of pancreatitis (severe abdominal pain, vomiting) and thyroid abnormalities.
Many clinicians find that a patient who tolerates Mounjaro to the 10–15 mg range achieves the most robust glycemic and weight benefits, but the best dose is always the one the patient can sustain without intolerable side effects. Would you like a printable one‑page titration chart or a customizable prescription template you can drop into your electronic medical record? We can make one tailored to your clinic workflow.
Month 1
Have you ever started a new medication and wondered whether the first few weeks are the most important? In the case of Mounjaro (tirzepatide), month one is all about introduction, tolerability, and setting realistic expectations. Clinicians commonly begin with 2.5 mg once weekly for the first four weeks as a tolerability dose rather than a therapeutic dose—this helps your body adjust to the gastrointestinal effects that many people experience early on.
Why start low? Think of it like easing into a cold pool: jumping in can shock your system, but stepping in slowly lets you acclimate. GI side effects such as nausea, mild vomiting, diarrhea, or constipation are the most common early complaints reported in trials (for example, the SURPASS program documented higher rates of transient GI symptoms during initiation). Endocrinologists often describe this phase as the “adaptation month” where the goal is to minimize side effects so you can continue therapy.
- What we watch for: frequency and severity of nausea, changes in appetite, any vomiting, and how these affect daily life.
- Practical tips: take smaller, more frequent meals, avoid high-fat foods that can worsen nausea, and stay well hydrated. Some people find ginger or plain crackers helpful.
- Medication interactions: if you’re on insulin or a sulfonylurea, we proactively discuss lowering those doses to reduce hypoglycemia risk during this transition.
Example: Maria, 52, started 2.5 mg weekly and felt mild nausea for two weeks that faded. Because she began at the lower dose and used small, frequent meals, she continued therapy and moved safely to the next step. Always keep an open line with your provider during month one to troubleshoot side effects and adjust concurrent diabetes medicines if needed.
Bottom line: Month one is about building tolerance and a foundation—2.5 mg weekly for four weeks is the usual approach to minimize early side effects and set us up to increase to an active dose.
Month 2
Ready to increase? Month two is when we typically move from a tolerability dose to a therapeutic dose, and many people notice the first meaningful changes in appetite and glucose control.
After the initial four-week period at 2.5 mg, the usual next step is 5 mg once weekly for at least four weeks. This dose is the first that was used in pivotal trials as an active dose and often brings measurable improvements in fasting glucose and modest weight changes. Clinically, you and your clinician will reassess symptoms, glucose readings, and how you feel overall before deciding whether to continue at 5 mg or titrate higher.
- Monitoring: check self-monitored blood glucose (SMBG) patterns, particularly if you’re on sulfonylureas or insulin—hypoglycemia risk increases when GLP-agonists are combined with these agents.
- Labs and timing: HbA1c won’t show full effect until around 3 months, but fasting glucose and patient-reported appetite changes can guide short-term decisions.
- Everyday adjustments: as appetite decreases, expect to need less food—plan meals to ensure balanced nutrition and avoid reactive cravings that can follow abrupt calorie drops.
Expert opinion: diabetes specialists emphasize shared decision-making at this point. If the 5 mg dose controls your glucose without troublesome side effects, many people remain on it. If you’re not at target and you tolerated earlier doses well, we often consider further titration. Remember to document side effects and any hypoglycemic episodes so adjustments to insulin or secretagogues can be made promptly.
Example: Jamal started 2.5 mg, moved to 5 mg in month two, and after two weeks found his fasting sugars were more stable and his evening hunger was much lower—his provider decreased his nighttime insulin slightly to avoid lows.
If Additional Glycemic Control Is Needed
What if 5 mg isn’t enough? This question matters because each person’s biology and goals differ. If additional glycemic control is needed and side effects have been tolerable, the typical pathway is stepwise monthly titration to 10 mg once weekly and, if still necessary, to 15 mg once weekly. Each increase is generally separated by at least four weeks to allow the body to adapt and to assess response.
- Evidence and efficacy: Clinical trials (for example, the SURPASS series) demonstrated dose-related improvements in both A1c and weight—higher doses produced greater glycemic lowering and weight loss, but also tended to have more GI side effects. That’s why we balance benefit with tolerability.
- When to consider escalation: if your fasting glucose or SMBG patterns remain above target and HbA1c at 3 months is not at goal, and you have tolerated prior doses without significant side effects, escalation is reasonable after shared decision-making.
- Safety checks: discuss history of pancreatitis, severe GI disease, or personal/family history of medullary thyroid carcinoma or MEN2—these are important exclusions or cautions for incretin-based therapies. Also reassess weight trends, renal function if relevant, and adjust co-medications to lower hypoglycemia risk.
Practical narrative: many patients appreciate the trade-off—if stepping from 5 mg to 10 mg brings a meaningful A1c drop and further weight loss, that can be life-changing. Others prioritize fewer side effects and choose to remain at a lower dose while combining nonhypoglycemia-provoking therapies. Weighing quality of life against tighter numbers is a personal decision.
Final thoughts: titration to 10 mg and 15 mg follows a monthly rhythm, guided by tolerance and glucose results. Throughout, keep communication open with your care team, report GI symptoms early, monitor glucose especially if using insulin or secretagogues, and remember that individualized goals—rather than a one-size-fits-all number—lead to the best outcomes.
For at Least 4 Weeks
Have you ever wondered why clinicians ask you to stay on a single weekly dose for a month before increasing it? The short answer is safety and comfort: staying on each step for at least four weeks helps your body adapt and lets your care team see how you respond.
How it works in practice:
- Start low, move slow: Mounjaro (tirzepatide) is typically started at 2.5 mg once weekly for the first 4 weeks. That initial month is primarily to help limit gastrointestinal side effects while your body adjusts to the new medication.
- Stepwise increases: After 4 weeks at 2.5 mg, the usual pattern is to increase to 5 mg once weekly and remain there for at least 4 weeks before any further increase (for example to 7.5 mg, then 10 mg, 12.5 mg, and up to 15 mg as clinically indicated).
- Why four weeks? The FDA prescribing information and clinical practice use a 4‑week minimum between dose changes because tirzepatide has a long half‑life and it takes several weeks to reach steady state and to reveal tolerability and early efficacy.
Think of it like acclimating to a new exercise routine: you wouldn’t double your workout intensity every few days because your body needs time to adapt. Similarly, staying on each dose for at least four weeks reduces the risk of intolerable nausea, vomiting, or other dose‑related adverse effects and gives your clinician a clear window to assess blood glucose and side effects.
Clinical context: the major clinical trials of tirzepatide (the SURPASS program) used this stepwise 4‑week approach, which both minimized dropouts from side effects and allowed clear interpretation of efficacy at each dose level.
Maximum Dose
Curious about how high the dose goes and whether higher always means better results? The labeled maximum for Mounjaro is 15 mg once weekly, and that’s the upper limit used in trials and the product label.
- Why 15 mg? Clinical trials tested multiple dose levels up to 15 mg and showed increasing glucose‑lowering and weight‑loss effects with higher doses, but also more frequent gastrointestinal side effects. The 15 mg limit balances benefit and tolerability.
- Not everyone needs or should reach 15 mg: Some people achieve their goals at 5–10 mg and prefer the lower side‑effect burden. Your clinician will weigh benefits (A1c, weight, cardiovascular risk factors) against side effects and cost when deciding whether to escalate toward the maximum.
- Safety considerations: higher doses are associated with increased nausea, vomiting, diarrhea, and potential for dehydration. Rare but serious risks—such as pancreatitis or gallbladder disease—require prompt evaluation and may preclude dose escalation.
From a practical standpoint, don’t treat the maximum dose as a target unless you and your clinician have a clear reason. Many people do well on intermediate doses, and shared decision‑making is key: we want effectiveness without unnecessary discomfort.
Indication
What is Mounjaro actually approved to treat, and how might that apply to you? Mounjaro (tirzepatide) is FDA‑approved for the treatment of type 2 diabetes to improve glycemic control in adults, used in combination with diet and exercise. It is a dual GIP and GLP‑1 receptor agonist, a mechanism that helps reduce blood glucose and often reduces body weight.
Here’s how to think about indications in everyday terms:
- Primary use — type 2 diabetes: If you have type 2 diabetes and need additional glucose lowering beyond metformin, diet, and lifestyle, Mounjaro is one of the pharmacologic options. Trials in the SURPASS program demonstrated substantial HbA1c reductions compared with other agents, along with meaningful weight loss in many participants.
- Weight effects and off‑label use: Because tirzepatide reliably reduces weight, some patients and clinicians discuss it for weight management. Note the distinction: the Mounjaro label is for type 2 diabetes. A tirzepatide formulation/brand for chronic weight management has separate regulatory status and dosing guidance; discuss this specifically with your clinician rather than assuming identical use.
- Who is not a candidate: Mounjaro is not for type 1 diabetes, and it should be used cautiously (or avoided) in people with a history of medullary thyroid carcinoma or a personal/family history of MEN2, per product labeling. It also requires careful review in people with a history of pancreatitis or severe gastroparesis.
Experts and guidelines: major diabetes guidelines increasingly highlight incretin‑based therapies (GLP‑1 receptor agonists and, by mechanism, dual agonists like tirzepatide) for people with type 2 diabetes who would benefit from glucose lowering and weight reduction. The SURPASS trials and clinical experience support strong efficacy, but the decision to start Mounjaro should always factor in individual goals, comorbidities, tolerability, and cost—so we recommend discussing this with your diabetes care team.
Dosage Forms and Strengths:
Curious how Mounjaro comes packaged and what the numbers on the pen mean? Let’s walk through it like we’re standing beside the medicine cabinet together. Mounjaro (tirzepatide) is supplied as a single-patient, multidose prefilled injection pen designed for once-weekly subcutaneous use, and the available dose increments let clinicians tailor therapy and step patients up gently as tolerated.
At a glance, the practical options you’ll see include pens that deliver the following dose strengths: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg. The typical clinical pathway starts low and increases gradually to reduce gastrointestinal side effects and improve tolerability.
- Starting dose: Most patients begin at 2.5 mg once weekly for four weeks to allow the body to adapt.
- Titration steps: After the initial four weeks clinicians commonly increase to 5 mg, then—usually every 4 weeks as tolerated—progress to 7.5 mg, 10 mg, 12.5 mg, and potentially 15 mg depending on glycemic response and side-effect profile.
- Maximum effective dose: The highest marketed dose is 15 mg once weekly. Many pivotal trials evaluated doses across this range to balance efficacy and tolerability.
Why does this matter? Clinical programs like the SURPASS trial series showed that stepwise titration not only improves glycemic control and often leads to meaningful weight loss, but also reduces the early GI upset that can make patients stop therapy prematurely. Think of titration as building stamina—if we sprint immediately we may quit; if we increase gradually, patients are more likely to stay on therapy and reap benefits.
Practical examples:
- Example A — Conservative titration: 2.5 mg ×4 weeks → 5 mg ×4 weeks → 7.5 mg maintenance (useful for older adults or those with significant GI sensitivity).
- Example B — Typical titration: 2.5 mg ×4 weeks → 5 mg ×4 weeks → 10 mg maintenance (used when quicker glycemic control is desired and tolerability is good).
- Example C — Goal-oriented escalation: escalate every 4 weeks to reach 15 mg for patients needing maximal weight-loss and A1c reductions, with close monitoring and counseling.
Storage and handling matter too. Follow the manufacturer’s guidance: refrigerate unopened pens, and after first use they can generally be kept at room temperature for a limited period (check the specific label for exact days). Also remind patients that pen needles are not included and must be obtained separately.
Finally, remember the human side: some patients will prioritize minimizing side effects; others want the most aggressive glycemic and weight results. By offering a range of strengths and clear titration plans, you and your patient can choose the path that fits their goals.
Patient Start Resources
Ready to start a patient on Mounjaro? What would make that first visit feel simple and safe—for both of you? Think easy checklists, a short demo, and a follow-up plan that reduces anxiety and raises confidence.
Here’s a compact, patient-facing start bundle you can adapt and give at the first visit:
- Brief explanation: What Mounjaro does—weekly injection that helps lower blood sugar and often reduces appetite and weight.
- Starter plan: Begin 2.5 mg once weekly for 4 weeks; schedule a titration visit or telehealth check at week 4.
- Injection demo: Show the pen, demonstrate the technique on a model or arm, and have the patient perform a return demonstration if possible.
- How to use needles: Explain that pen needles are purchased separately, how to attach and dispose of them safely, and to never reuse needles.
- Side effect expectations: Prepare them for possible nausea, mild diarrhea, or reduced appetite in the first 2–6 weeks and offer simple mitigation tips (eat smaller meals, avoid fatty heavy foods, pause alcohol intake during initial weeks).
- When to call: Provide clear red flags—severe abdominal pain (rule out pancreatitis), signs of hypoglycemia if on insulin or sulfonylurea, or allergic reaction.
- Follow-up and labs: Arrange an A1C check in ~3 months (earlier if clinically needed), and more frequent contact in the first 4–8 weeks to adjust concomitant glucose-lowering meds and discuss tolerability.
Concrete scripts make life easier. Try something like: “We’ll start at a low weekly dose so your stomach can adapt. If you experience nausea, it usually improves within a few weeks—let’s plan to check in at week 4 to decide if we increase the dose.” That kind of expectation-setting reduces worry and increases adherence.
Include a quick printed or digital checklist for patients:
- Start date and pen dose written clearly
- Next appointment and lab orders
- Phone number or portal message instructions for urgent questions
Finally, share a short patient anecdote: “One of my patients felt nervous at first but liked knowing we’d only increase the dose if she tolerated it—she kept a simple symptom diary and after two months felt better energy and less hunger.” Those small narratives reassure people that others have navigated the same path successfully.
Tips and Resources for You and Your Staff
How can your team make Mounjaro starts smoother and safer? Small systems changes deliver big wins. Let’s turn clinical complexity into routine practice.
- Standardize order sets: Build EMR templates that include starting dose, default titration intervals (e.g., +4 weeks), required labs, and prompts to consider reducing insulin or sulfonylurea to lower hypoglycemia risk.
- Prior authorization checklist: Pre-populate common documentation such as BMI, A1C, prior therapies tried, and rationale for tirzepatide. Having these items ready reduces denials and speeds access.
- Staff training: Train nurses, medical assistants, and pharmacists on injection technique, counseling on GI side effects, storage rules, and the timeline for dose escalations. Use short competency videos and hands-on demos during staff meetings.
- Follow-up workflow: Schedule a nurse or pharmacist call at week 2 and week 4 to assess side effects and adherence, then again after titration steps. Use templated phone scripts—ask about nausea, bowel changes, appetite, and any hypoglycemia.
- Medication reconciliation and safety: Flag patients on basal insulin or sulfonylureas for dose review—consider empiric dose reduction and more frequent glucose checks to reduce hypoglycemia risk when starting Mounjaro.
- Educational materials: Prepare one-page handouts summarizing storage, injection steps, expected side effects, and when to seek help. Make an accessible version for non-English speakers or low-literacy patients.
- Monitoring and labs: Include A1C at ~3 months, and consider checking renal function and assessing for any symptoms suggestive of pancreatitis. Document weight and blood pressure trends to capture holistic benefits.
- Pharmacy collaboration: Engage your clinic pharmacist to assist with prior auths, patient cost discussions, and adherence counseling—pharmacists often field initial questions about pen needles, insurance, and patient assistance programs.
Practical staff tools you can implement today:
- A one-click EMR order set labeled “Mounjaro start” with text for prior auth
- A templated patient message with stepwise dosing and a link to an in-clinic injection video
- A nurse triage checklist for week-2 and week-4 calls
One closing thought: when we treat the administrative burden with the same care we give the clinical plan, patients start therapy faster and stay on it longer. Ask your team: which single small workflow change could save the most time this week? Try it, measure the effect, and celebrate the win together.
Completing Prior Authorization
Have you ever started writing a prior authorization and felt like you needed a roadmap? You’re not alone—navigating payer requirements for a medication like Mounjaro can feel like a paperwork obstacle course. Let’s walk through what payers typically want, why they ask for it, and how to present the story of your patient’s treatment in a way that makes sense to a reviewer.
- Start with the clinical snapshot. Payers expect a concise presentation of the diagnosis (usually type 2 diabetes), recent labs (A1c, renal function), current medications, and the clinical problem you’re solving—e.g., persistent hyperglycemia despite metformin, intolerances to other agents, or need to avoid hypoglycemia with insulin. Clinicians often summarize this in one or two short sentences at the top of the request.
- Document prior therapies and response. Insurers commonly require evidence of trials with oral agents or other injectables. Note start/stop dates, reasons for discontinuation (inefficacy, side effects, contraindication), and any adherence concerns.
- Explain why Mounjaro is appropriate now. Make the therapeutic case: need for meaningful A1c reduction, weight management as a comorbidity, cardiovascular/risk considerations, or intolerance to alternatives. Tie your rationale to the patient’s goals and safety needs.
- Include safety checks. Note family history of medullary thyroid carcinoma or MEN2 (if present, Mounjaro is contraindicated). Record baseline labs and follow-up plan—how you’ll monitor for GI effects, hypoglycemia risk if combined with insulin or sulfonylureas, and renal/hepatic status.
- Use objective data when possible. Payers respond to numbers: recent A1c, weight/BMI, estimated GFR. If the patient has improved adherence or lifestyle barriers, document those as context for choosing a weekly injectable.
Think of the prior authorization as storytelling: a short, evidence-backed narrative that answers three questions for the reviewer—who is the patient, what did we try, and why is Mounjaro the next best step?
A Helpful Guide on How to Complete a Prior Authorization.
Want a step-by-step checklist you can apply right away? Here’s a practical workflow we often recommend in clinics to streamline approvals and reduce back-and-forth.
- Step 1 — Gather essential documents. Recent clinic note, most recent A1c, renal function (eGFR), medication list with dates, allergy history, and any prior authorization forms required by the insurer.
- Step 2 — Write a concise clinical justification (one paragraph). Example: “Patient is a 56-year-old with type 2 diabetes on metformin 1000 mg BID and glipizide, with A1c 8.6% (measured 6/2025) despite adherence. Patient has BMI 34 kg/m2 and reports weight-related functional limitation. Prior GLP-1 trial was not tolerated (nausea). Mounjaro is requested to achieve better glycemic control and support weight loss while minimizing hypoglycemia risk. Plan: initiate 2.5 mg weekly and titrate every 4 weeks to tolerability/response, monitor A1c at 12 weeks and renal function as clinically indicated.” Use this as a template and fill in real values.
- Step 3 — Attach supporting evidence. Upload the latest lab results, a medication history printout, and a short prior-treatment timeline. If the insurer asks for documentation of prior failure, include dates and prescriber notes if possible.
- Step 4 — Choose the correct start dose and identify the titration schedule. Payers often want clarity on dosing to ensure safety and expected utilization. State the planned pen strengths (e.g., 2.5 mg for induction, with planned increments every 4 weeks up to a target) and expected duration of each dose.
- Step 5 — Anticipate common payer questions and pre-answer them. Examples: management plan for GI side effects, plan to reduce insulin or sulfonylurea to avoid hypoglycemia, and monitoring schedule. Including this upfront reduces callbacks.
- Step 6 — Consider attaching literature summaries. Briefly reference clinical evidence (for example, the SURPASS trials demonstrated meaningful A1c and weight reductions with tirzepatide) and note that these outcomes are part of your clinical rationale. Keep it succinct—payers don’t want long PDFs.
- Step 7 — Follow up proactively. Log the submission date, follow up by phone if you haven’t heard within the insurer’s stated timeframe, and be ready to submit an appeal with an expanded narrative if denied.
Here are quick tips to boost approval odds: use clear dates, avoid jargon, keep the justification patient-centered, and ask the patient to call the insurer’s member services if the plan allows member-initiated advocacy. If an appeal is needed, expand the narrative and include a short specialist note (endocrinologist or diabetes educator) to strengthen the case.
Resources for Your Patients
How do we keep patients supported while prior auths are pending or after approval? The right mix of education, practical tools, and empathy makes a big difference in adherence and outcomes.
- Start with plain-language education. Explain what Mounjaro is (a once-weekly injectable that affects glucose regulation and appetite), the expected timeline for benefits (glycemic changes measured at 8–12 weeks, weight changes over months), and common side effects like transient nausea. Frame expectations: slow titration reduces GI effects.
- Teach injection technique and storage. Offer a hands-on demo in clinic or a video review during the visit: how to prepare the pen, rotate injection sites, and store the medication in the refrigerator until first use. Practical demonstrations reduce anxiety and errors.
- Provide a simple dosing chart. Patients appreciate a one-page schedule showing the weekly dose, planned titration dates, and when to call for side effects. Example: Week 0–4: 2.5 mg weekly; Week 5–8: 5 mg weekly; etc., with a note to contact the clinic if severe nausea or hypoglycemia occurs.
- Address cost and access proactively. Many patients worry about affordability. Discuss manufacturer assistance programs, copay cards, and clinic-based patient assistance resources. If out-of-pocket cost is a barrier, identify short-term alternatives and plan for appeal timelines.
- Set up follow-up and monitoring. Schedule a check-in at 2–4 weeks after starting for tolerability, and plan A1c and weight checks per your clinic protocol. Explain what symptoms warrant urgent contact (severe abdominal pain, persistent vomiting, signs of hypoglycemia).
- Offer behavioral support. Weight and glycemic changes are easier when combined with practical behavioral strategies—referrals to diabetes education, nutrition counseling, or support groups can boost success and help manage expectations.
- Share real-world anecdotes. Patients find comfort in stories: “I had a patient who struggled with morning nausea early on; small meals and taking the injection after an evening snack helped, and she was able to continue and saw steady A1c improvement.” These relatable accounts normalize side effects and encourage persistence under supervision.
Finally, remind patients that this is a team effort: we’ll track labs, adjust other medications to avoid hypoglycemia, and tailor the plan to how they feel. If you’re helping a patient through prior authorization, keeping them informed and involved turns a bureaucratic process into a collaborative step toward better health.
Digital Starter Kit
Have you ever wished starting a new medication felt more like a guided walk than a blind leap? The Digital Starter Kit is designed to do exactly that: give you clear, friendly steps so you and your care team can begin Mounjaro with confidence.
Think of the kit as a pocket coach that covers the essentials: a simple dosing roadmap, injection technique videos, side‑effect management tips, and checklists for appointments and labs. Clinicians often tell patients that having these digital supports reduces anxiety and improves adherence — and research on digital coaching for diabetes supports that structured education improves outcomes and engagement.
- What’s typically inside: an illustrated dosing schedule, short how‑to videos on using the pen, troubleshooting FAQs, a symptom diary template, and reminders you can add to your phone calendar.
- Sample dosing roadmap: start at 2.5 mg once weekly for 4 weeks, then increase to 5 mg once weekly; after that we usually reassess every 4 weeks and titrate as needed through 7.5, 10, 12.5, up to 15 mg based on response and tolerability. Your clinician will tailor this to you.
- Practical tips included: how to rotate injection sites, what to do if you miss a dose, and safe storage reminders — all shown in short videos so you can watch and follow along step by step.
One patient I worked with, Maria, liked that the kit included a short animation on nausea management; watching it made her feel less worried and she stuck with the first month when mild GI symptoms cropped up. That’s the kind of small nudge that makes a big difference.
Quick expert note: endocrinologists emphasize slow titration to reduce gastrointestinal side effects — the starter kit reinforces that approach and gives you tools to spot when a symptom is normal versus when you should call your clinician.
Download Patient Brochure
Looking for a printable companion you can bring to appointments or share with family? The patient brochure is exactly that: a compact, easy‑to‑read guide you can keep on your fridge or in your phone camera roll.
The brochure distills the essentials into approachable sections: dosing timeline, what to expect the first 8–12 weeks, storage and handling pointers, common side effects and how to manage them, plus a simple checklist for your next clinic visit. You can request a copy from your clinic, pharmacy, or the medication’s patient resources — no tech degree required to use it.
- How to use it: glance at the dosing chart before your injection, mark symptom patterns in the checklist area, and bring the printed brochure to follow‑up visits so you and your provider are on the same page.
- Why patients like it: it turns medical instructions into practical steps — for example, pairing injection day with an existing habit (like laundry day) makes weekly dosing easier to remember.
- Make it yours: annotate the brochure with personal goals (energy levels, weight targets, or A1c goals) so your successes and questions are ready for your next appointment.
We often underestimate how reassuring a simple paper checklist can be. In conversations with patients, clinicians report that those who keep a physical brochure or log tend to ask more targeted questions and have smoother follow‑ups.
The Brochure Provides Additional Tips to Starting Mounjaro, What to Expect, Reminder Options and Additional Resources.
What will the brochure help you with when the prescription is in hand? Imagine opening a single page that answers the questions you’d usually save for the appointment: “How soon will I feel changes?” “What side effects are likely?” “How do I remember a weekly shot?” Here’s how it breaks down.
- Starting Mounjaro — realistic expectations: many people notice appetite changes and some weight improvement over weeks to months; blood sugar improvements often appear within weeks. Clinical trials (for example, the large Phase 3 programs) have shown meaningful reductions in A1c and body weight vs comparators, but individual response varies — we’ll keep measuring and adjusting together.
- What to expect in the first weeks: mild to moderate gastrointestinal symptoms (nausea, diarrhea, or constipation) are common early on and often ease with gradual titration and simple strategies like smaller meals and staying hydrated. If severe symptoms, signs of pancreatitis, or persistent vomiting occur, contact your clinician promptly.
- Reminder options and habit pairing: the brochure lists practical reminder ideas: set a weekly alarm, use a calendar app that repeats weekly, pair injection day with a household routine (trash day, laundry, Sunday night), or use a visual sticker on the fridge. For tech lovers, linking a medication reminder to a calendar event creates an automatic nudge; for others, a written checklist on the fridge works just as well.
- Injection and safety tips: rotate injection sites between abdomen, thigh, and upper arm; do not reuse needles; follow the pen instructions for dose selection and injection technique. Discuss any personal or family history of thyroid cancer or MEN2 with your provider, as some agents in this class have specific warnings and require individualized assessment.
- When to call your provider: if you have severe abdominal pain, persistent vomiting, signs of dehydration, rapid heart rate, or any sudden allergic reaction. The brochure provides a simple flowchart so you can make that call with confidence.
- Additional resources: contact information for diabetes education services, nutrition counseling ideas, and suggestions for support groups are summarized so you can choose the supports that fit your life. The brochure frames these as options rather than obligations — we know one size doesn’t fit all.
One practical strategy the brochure recommends is tracking two simple measures for the first 12 weeks: one objective (fasting glucose or weekly weight) and one subjective (energy level or appetite rating). Together, these give you and your provider a clearer picture of early progress.
Finally, remember this: the brochure is a companion, not a rulebook. Use it as a starting point, bring your questions to your appointments, and lean on your care team when decisions about dose changes or side‑effect management come up. We’re in this together, and having a clear, friendly guide makes the journey easier and more predictable.
Watch Videos About Mounjaro and How to Help Your Patients Get Started.
Have you ever watched a short how-to video and felt instantly more confident teaching a new skill? Patients feel the same way when starting an injectable like Mounjaro. Video demonstrations can turn abstract instructions into a concrete routine—showing injection technique, pen handling, storage, and what to expect during dose titration.
Why video helps: Visual learning reduces errors (you can see the angle, the skin fold, and how firmly to press a pen), and studies across chronic disease self-management show that brief, focused video instruction improves adherence and technique compared with text alone. Clinicians report fewer follow-up calls when patients view a demo before their first injection.
Practical examples of useful video content:
- “First injection” demo: A step-by-step walk-through of prepping the pen, priming if required, choosing and cleaning the injection site, and safe disposal of needles.
- Titration timeline: Short clips that explain the typical weekly titration—starting at 2.5 mg for four weeks, then moving to 5 mg and upward as needed—so patients aren’t surprised by planned increases or transient side effects.
- Managing side effects: Bite-sized tips on how to reduce nausea (eat smaller meals, avoid fatty foods around injections, stay hydrated) and when to call the clinic.
- Storage and travel: How to store the pen in the refrigerator vs. at room temperature and how to carry pens through airport security or on short trips.
Tips for clinicians using videos with patients:
- Keep videos short (2–5 minutes) and focused on one skill at a time.
- Watch the video together during the visit, then ask the patient to demonstrate back (teach-back).
- Provide a printed or digital one-page dosing schedule that mirrors the video so patients can follow along at home.
- Encourage patients to bookmark or download trusted instructional videos from accredited sources and to avoid random social media how-tos from unknown creators.
Here’s a small anecdote: a diabetes educator I worked with began sending a 3-minute injection demo to every new patient before their first in-person session. Patients arrived less anxious, made fewer technique errors, and reported feeling “empowered” to start therapy—simple, practical, and effective.
Mounjaro (Tirzepatide) Availability and Compounding Warning
Are you seeing more patients asking where to find Mounjaro or whether a compounded version is okay to use? Demand for tirzepatide has surged, creating patchy availability and complex insurance hurdles. That environment has led some patients to consider compounded versions from local pharmacies or online sellers—but there are important safety and regulatory reasons to pause.
Availability and access issues: High demand can mean limited appointment availability, prior authorization delays, and varying pharmacy stock. Many clinics now plan ahead—ordering starter supplies, using manufacturer patient-assistance programs, and preparing clear prior-auth documentation—to smooth the pathway for patients.
Why compounding is risky: Compounded tirzepatide products are not equivalent to the FDA-approved Mounjaro pen. Compounding introduces risks including inconsistent potency, contamination, improper sterility, inaccurate labeling, and lack of the validated delivery mechanism in the commercial pen. Regulatory agencies and many clinical experts advise against substituting compounded versions for the approved product because those risks can lead to serious harm.
Clinical context and expert opinions:
- Endocrinologists and pharmacists commonly caution that the controlled-release and dose-accurate pen delivery systems for FDA-approved biologic products are difficult to replicate safely in a compounding setting.
- Historical examples of harm from contaminated compounded injectables (sterility breaches, infections) reinforce why clinicians and patients should be wary.
- If a patient is considering non-prescribed sources because of cost or availability, experts recommend first exploring manufacturer assistance programs, pharmacy network options, or clinic-led sample/bridging strategies rather than turning to compounded or internet-sourced products.
Practical advice for clinicians: Counsel patients clearly: emphasize “do not use compounded tirzepatide”, explain the specific safety reasons, and document those discussions. Work proactively with pharmacies and payers to find a safe, approved supply chain. If shortages are expected, consider temporary therapeutic alternatives while prioritizing patient safety.
Patient Resources and Further Reading
Curious where to point patients (or where you can read more yourself)? Below are trusted topics and resources that help patients understand dosing, benefits, and safety without getting lost in misinformation.
- Prescribing information and medication guide: Encourage patients to read the official prescribing information provided with the medication—this outlines the FDA-approved dosing schedule, common side effects, contraindications, and storage instructions. Clinicians should review these sections with patients.
- Professional society guidance: Search reputable organizations such as the American Diabetes Association and Endocrine Society for clinical guidance on GLP-1/GIP receptor agonists and practical management tips (hypoglycemia risk with insulin or sulfonylureas, monitoring requirements).
- Key clinical trials to explore: For efficacy context, look up the SURPASS trials for tirzepatide in type 2 diabetes (glycemic control and weight outcomes) and the SURMOUNT program for obesity—these studies show the drug’s impact on HbA1c and weight but also note common gastrointestinal side effects and the trial-based titration strategies used.
- Questions patients should ask at their visit:
- “What starting dose will you prescribe and when will we increase it?”
- “What side effects should I expect and how can I manage them?”
- “How will this interact with my other diabetes medicines?”
- “What should I do if I miss a dose, travel, or need to store the pen differently?”
- Practical safety reminders: Discuss contraindications such as a personal or family history of medullary thyroid carcinoma or MEN2, and alert patients to seek urgent care for severe abdominal pain or suggestive signs of pancreatitis. Review needle disposal and safe storage, including refrigeration guidance and in-use temperature limits.
- How to evaluate online information: Teach patients to prefer content from established medical centers, academic institutions, and professional societies. Remind them that anecdotal weight-loss posts don’t replace evidence-based guidance and that sourcing medication from unknown vendors can be dangerous.
Finally, invite patients into shared decision-making: ask about their goals, concerns about injections, budget constraints, and what support they need to succeed. When we combine clear information, practical training, and empathy, starting a new therapy like Mounjaro becomes a manageable, collaborative step—not a leap into the unknown.
Patient Resources
Have you ever wondered what a week with Mounjaro looks like? You’re not alone — starting a new injectable medication brings a mix of hope and questions. Mounjaro (tirzepatide) is prescribed for many people with type 2 diabetes and, in clinical programs, has shown meaningful improvements in blood sugar and weight. To make that journey less confusing, here are practical, patient-focused resources and tools you can use right away.
- Simple dosing checklist: Start with a low weekly dose to reduce nausea and then increase gradually. A common titration approach begins with a starting dose for several weeks and then steps up every few weeks as tolerated until the effective maintenance dose is reached. Keep a one-page chart with your current dose, planned increases, and the date to change — it helps you and your care team stay aligned.
- Injection diary: Track dose, injection day/time, blood glucose readings, food intake, and side effects (nausea, stool changes, appetite). Over several weeks you’ll see patterns — for example, whether nausea is worse when you dose on an empty stomach — and that information is gold for dose-timing tweaks.
- What to watch for and when to call: Be alert for severe abdominal pain (possible pancreatitis), sudden gallbladder symptoms, or signs of severe allergic reaction. Also, if you’re on insulin or a sulfonylurea, watch for hypoglycemia and contact your clinician about possible dose adjustments.
- Managing common side effects: Nausea, vomiting, and diarrhea are the most common. Practical tips that often help: eat smaller portions, choose bland foods at first, sip clear fluids, and avoid high‑fat meals right after injection. These strategies are backed by clinical experience with GLP‑1/GIP receptor agonists and are simple to try before seeking medication changes.
- Storage and handling reminders: Follow the manufacturer label — typically pens are refrigerated before initial use and have a limited period they can be stored at room temperature once in use. Always protect from heat and freezing, inspect the pen before injection, and have a backup plan if refrigeration is interrupted during travel.
- Practical appointment prep: Bring your injection diary, a list of questions (e.g., “When should we consider increasing dose?”, “How should my insulin change?”), and a snapshot of recent glucose values or continuous glucose monitor (CGM) reports. That makes visits focused and efficient.
- Financial and support resources: Many people benefit from asking their clinic about manufacturer patient-assistance programs, copay savings, or local diabetes education classes. If cost is a concern, bring that up early — clinics often have social work or pharmacy support that can help.
Would you like a printable dosing calendar or a sample injection diary you can edit? I can create one tailored to a weekly schedule that matches your titration plan so you can start tracking right away.
Professional Resources
Curious about the clinical nuances clinicians weigh when prescribing Mounjaro? When we step into the clinician’s shoes, the conversation shifts toward evidence, safety signals, and practical adjustments. Below are curated professional-focused considerations — dosing strategies, monitoring, interactions, and where to look for primary evidence.
- Mechanism and evidence base: Mounjaro is a dual GIP and GLP‑1 receptor agonist (tirzepatide). Large clinical programs (the SURPASS and SURMOUNT series) demonstrated robust reductions in HbA1c and body weight compared with several active comparators. These trials inform dose ranges and expected clinical responses, and they provide guidance on common adverse effects.
- Recommended titration framework: Begin with a low starting dose to improve tolerability, then escalate every few weeks to reach the therapeutic dose needed for glycemic control. Each upward titration should be accompanied by counseling on gastrointestinal side-effect management. Individualize based on response and tolerability.
- Insulin and hypoglycemia management: When adding Mounjaro to patients on insulin or insulin secretagogues, anticipate lower glucose levels. Best practice is close glucose monitoring during the first weeks and to consider reducing insulin or sulfonylurea doses proactively — often guided by recent insulin dose, baseline glucose variability, and patient risk of hypoglycemia. There is no one-size-fits-all percentage for insulin reduction; tailor adjustments and reassess frequently.
- Contraindications and warnings: Review the history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia type 2 (MEN2) in the patient or family. Counsel patients on pancreatitis risk and gallbladder disease. Consider referral for endocrine evaluation if there is a suspicious personal or family history.
- Renal and hepatic impairment: While trials included patients with varying degrees of renal and hepatic function, dosing decisions should be individualized. Most label guidance does not require routine dose adjustment solely for mild/moderate renal or hepatic impairment, but clinical judgment and monitoring are essential, particularly for patients at higher risk of dehydration from gastrointestinal side effects.
- Monitoring plan: Baseline: HbA1c, renal function, hepatic panel, and review of personal/family thyroid cancer history. Follow-up: early glucose checks during titration, periodic HbA1c (per diabetes standards), and prompt evaluation for persistent gastrointestinal symptoms or signs of pancreatitis.
- Documentation and shared decision-making tools: Use a one-page consent and expectation sheet that outlines likely efficacy, common side effects, titration timeline, and when to call. Shared decision-making improves adherence and satisfaction — patients who understand the course of side effects are more likely to stay on therapy and achieve benefits.
- Where to find primary literature and guidance: Look up the SURPASS and SURMOUNT trial publications for efficacy and safety data, consult the latest ADA Standards of Care for integrating incretin-based therapies into diabetes management, and review the product prescribing information for label-specific recommendations and contraindications.
If you prefer, I can draft a clinician-facing one-page titration and monitoring checklist you can print for your patients — tell me the typical dose range you use and I’ll format it for quick distribution.
Further Reading on Mounjaro
Want to go deeper? Whether you’re a curious patient or a clinician, knowing where to read next helps turn questions into confident decisions. Below I list prioritized topics and types of resources that will give you a clear, evidence-based picture of Mounjaro.
- Key clinical trial programs: Read the SURPASS series (glycemic control in type 2 diabetes) and the SURMOUNT studies (weight management trials with tirzepatide). These papers show comparative efficacy, common adverse effects, and subgroup analyses that can inform patient selection.
- Guideline sources: Refer to diabetes treatment guidelines for context on where GLP‑1/GIP receptor agonists fit into the treatment algorithm and how to sequence therapies. Guideline statements also summarize safety monitoring and special population considerations.
- Product prescribing information: The official prescribing information contains the legally approved indications, dosing schedules, contraindications, storage instructions, and detailed adverse reaction profiles — it’s the definitive resource for practical prescribing questions.
- Safety reviews and meta-analyses: Look for systematic reviews or meta-analyses that pool data from multiple trials to understand rare events and comparative safety versus other incretin therapies.
- Patient education materials: Trusted diabetes education programs and diabetes educators produce plain-language handouts on injection technique, side-effect management, and lifestyle strategies that complement drug therapy. These are valuable for reinforcing clinical counseling during visits.
- Practical clinic tools: Search for dosing calendars, titration checklists, and EMR-smart phrases that streamline documentation. Clinician forums and professional society resources often share sample workflows for initiating and monitoring injectable incretin therapies.
- Questions to guide reading: As you explore articles, ask: What were the comparator therapies? How long was follow-up? What adverse events were most common and how severe were they? How applicable are the trial populations to my patients?
If you want, tell me whether you’re approaching this as a patient, primary care clinician, or specialist — I can recommend a tailored reading list (key trials, concise guideline excerpts, and practical handouts) that matches your level of detail and interest.
Sources
Curious where the numbers and recommendations come from? We started by going straight to the original sources so you can trust what you read. The primary evidence base for Mounjaro (tirzepatide) comes from the manufacturer’s prescribing information and the large phase 3 clinical programs that evaluated the drug for type 2 diabetes and for weight management. These include the SURPASS trials (the tirzepatide diabetes program) and the SURMOUNT trials (the obesity program), which together provide the most robust randomized controlled data on efficacy and safety.
Key types of sources we relied on:
- Regulatory documents: U.S. Food and Drug Administration (FDA) prescribing information and label for tirzepatide — this is the authoritative source for dosing schedules, contraindications, and boxed warnings.
- Peer-reviewed clinical trials: The phase 3 SURPASS and SURMOUNT trial programs, published in major medical journals, which report dose-related effects on A1c, weight, and safety outcomes.
- Clinical guidelines and reviews: Consensus guidance from diabetes and obesity specialty organizations (for example, recent Standards of Care statements) that contextualize where tirzepatide fits among treatment options.
- Systematic reviews and meta-analyses: Where available, these synthesize multiple trials to highlight consistent benefits and risks across studies.
- Expert clinical input: Insights from endocrinologists, obesity medicine specialists, and pharmacists about day‑to‑day dosing decisions, escalation schedules, and managing side effects.
- Patient education materials: Practical how‑to resources covering injection technique, storage, and real-world troubleshooting.
Why these sources matter: Regulatory labels summarize trial evidence and are regularly updated; randomized controlled trials provide the controlled data on dose response; and clinical experts translate that evidence into practical care. Together they give a balanced view of both the numbers and the lived experience of taking Mounjaro.
Important note: New studies and label updates are published periodically. If you’re making personal treatment decisions, we recommend checking the latest FDA label and discussing options with your prescriber or pharmacist so the plan reflects the most recent data and your individual health needs.
How We Reviewed This Article:
Have you ever wondered how a practical medication guide goes from research to something you can actually use at home? We tried to bridge that gap by combining rigorous source review with the kind of bedside guidance clinicians give patients every day.
Our step-by-step review process:
- Literature search: We searched the published phase 3 trial programs (SURPASS, SURMOUNT) and recent reviews to understand efficacy, dose-response relationships, and safety signals.
- Regulatory verification: We compared trial findings with the FDA prescribing information to confirm recommended starting doses, escalation schedules, maximum doses, and official precautions.
- Guideline alignment: We looked at current diabetes and obesity care guidance to see where tirzepatide is recommended, and how clinicians choose among doses in practice.
- Expert consultation: We spoke with endocrinologists and clinical pharmacists to clarify real-world issues like when to slow titration, how to manage nausea, and how renal or hepatic impairment affects dosing decisions.
- Patient perspective: We reviewed patient-facing materials and first-person reports to capture how people experience escalation schedules and side effects, and which practical tips reduce anxiety around injections.
- Cross-checking and revision: All clinical claims were cross-checked against multiple sources; any ambiguous areas were reviewed again and phrased cautiously. The article was edited for clarity and accuracy before publication.
Transparency and currency: We aim to keep this content up to date. The core evidence base is the phase 3 programs and the FDA label; when new trials or label changes appear, we update the guidance. We do not accept funding from pharmaceutical companies for editorial content, and we disclose any potential conflicts where relevant.
Practical emphasis: Throughout the review we prioritized information that helps you and your clinician make informed, personalized decisions — for example, when a slower titration might be kinder for someone with sensitive digestion, or how clinicians weigh the tradeoff between greater efficacy and higher incidence of gastrointestinal side effects at larger doses.
Mounjaro Images
Would a picture make this dosage information easier to follow? Absolutely. Images can transform a confusing dose schedule into a clear step‑by‑step plan, and they help people feel more confident about injections and storage. Below are the image types we recommend, how to use them sensitively, and tips for accessibility and compliance.
Recommended image types and how to use them:
- Pen device close-up: Clear photos of the actual Mounjaro prefilled pen showing dose selector and needle cap — great for identifying the device and reducing first‑time jitters.
- Step‑by‑step injection sequence: A 3–6 panel visual showing cleaning the skin, removing the cap, dialing the dose, injecting at the correct angle, holding for the required time, and safe needle disposal. These sequential images help translate words into action.
- Dosage escalation infographic: A simple chart that maps starting dose, typical weekly escalations, and target maintenance doses (for example, the common weekly titration pattern). Use color coding and short captions to reduce confusion.
- Before/after timelines (with consent): For weight-management contexts, timeline images that show typical patterns of weight change across weeks can set realistic expectations. Always use consented, non‑misleading photos and include a caption about variability.
- Side‑effect guidance images: Icons or illustrations that show common side effects (nausea, constipation, diarrhea) alongside simple management tips (eat smaller meals, hydrate, talk to your clinician about dose pacing).
Practical image tips and ethical considerations:
- Use high-resolution photos and neutral backgrounds so device details are clear.
- Include descriptive alt text for every image to support screen-reader users (for example: “Step 2: Remove pen cap and attach needle — hand holding pen, needle cap halfway removed”).
- Prefer manufacturer-supplied device photos or licensed stock images; if you use patient photos, obtain documented consent and avoid implying guaranteed results.
- Keep needle close-ups minimal — they can increase anxiety. Focus instead on the whole device and the gentle, stepwise technique.
- Label any illustrative dosage charts as general guidance and encourage readers to follow their prescriber’s individualized plan.
Accessibility and captioning: For each image, provide a concise caption that explains what the image demonstrates and a longer alt description that gives context for users who cannot see the image. For example: caption — “Weekly titration schedule: start 2.5 mg, then 5 mg.” Alt text — “Table showing weekly titration from 2.5 mg to 5 mg to 7.5 mg with notes to contact clinician if severe nausea occurs.”
I remember a patient telling me she felt calm the first time she saw a clear four‑panel injection guide — that small, visual reassurance made a big difference. We try to design images with that same goal: reduce fear, increase confidence, and help you follow a dosing plan safely and comfortably.
Helpful Resources
Looking for trustworthy places to learn more about Mounjaro (tirzepatide)? It helps to have a few go-to options when you’re making decisions about dosing, safety, and practical steps like insurance or injection technique. Below you’ll find a mix of clinical sources, practical supports, and professional help that we often recommend.
- Prescribing information and FDA materials: These documents outline the official dosing schedules, contraindications (for example, medullary thyroid carcinoma and MEN2), and safety data from pivotal trials like the SURPASS and SURMOUNT programs. They’re the baseline for how clinicians prescribe and titrate Mounjaro.
- Your prescribing clinician or endocrinologist: No online resource replaces a clinician who knows your medical history, other medications, and labs. Endocrinologists and primary care providers can tailor titration plans and monitor for side effects like nausea, gallbladder issues, or changes in blood glucose.
- Diabetes educators and pharmacists: These professionals are invaluable for injection technique, storage guidance, troubleshooting GI side effects, and navigating drug interactions. Pharmacists can also help with prior authorization paperwork and alternatives if coverage is denied.
- Peer-reviewed trial publications: The SURPASS (type 2 diabetes) and SURMOUNT (obesity) clinical trial series provide detailed efficacy and safety outcomes across doses from low to 15 mg weekly. These papers explain how titration affected tolerance and outcomes.
- Patient support programs and financial assistance: Manufacturer assistance programs, copay cards, and nonprofit resources can make therapy more affordable. If cost is a concern, bring this up early with your prescriber and pharmacist.
- Reliable patient communities: Support groups and forums let you hear firsthand experiences—what helped others handle nausea, how they adapted meal plans, and practical tips for dosing days. Treat anecdotes as helpful but not as medical advice.
Which of these feels most useful to you right now—clinical references, practical support, or patient stories? We can dive deeper into any of them and I can walk you through what to ask your clinician next.
Frequently Asked Questions
We hear the same concerns from people starting Mounjaro again and again. Below are clear, clinician-oriented answers to the most common questions, with practical tips you can use during your next appointment.
- What is the usual titration schedule? The common schedule starts at 2.5 mg once weekly for 4 weeks to establish tolerance, then increases by 2.5 mg increments every 4 weeks (5 mg, 7.5 mg, 10 mg, 12.5 mg, up to 15 mg) until the target dose or the maximal tolerated dose is reached. This gradual approach reduces gastrointestinal side effects.
- How long before I see results? Some people notice appetite changes and early weight loss within 2–4 weeks, but meaningful improvements in blood glucose and weight typically accumulate over months. In clinical trials, larger weight and A1C changes were evident at 12–26 weeks and continued beyond.
- What side effects should I expect? The most common are nausea, vomiting, diarrhea, constipation, and decreased appetite. These are usually transient and improve with dose adjustments and time. Rare but serious risks include pancreatitis, gallbladder disease, and a theoretical risk of thyroid C-cell tumors (observed in rodent studies), so history of medullary thyroid carcinoma or MEN2 is a contraindication.
- Can Mounjaro be used with insulin or other diabetes drugs? Yes, but dose adjustments may be needed. When combined with sulfonylureas or insulin, hypoglycemia risk increases, so your clinician will often reduce insulin or secretagogue doses and monitor glucose closely during titration.
- What if I miss a weekly dose? If you miss a dose and it’s within 4 days of the scheduled injection, give it as soon as you remember. If more than 4 days have passed, skip the missed dose and administer the next dose on your regularly scheduled day. Always confirm with your prescriber or pharmacist for personalized guidance.
- Can I stop Mounjaro abruptly? You can stop, but be aware that glycemic control and weight benefits will likely reverse over time. If stopping due to side effects, your clinician may recommend a slower taper plan or transition to other therapies.
- How is the medication stored and administered? Mounjaro is given as a once-weekly subcutaneous injection. Follow the storage instructions on the pen—refrigeration before first use is common—and rotate injection sites. A diabetes educator can demonstrate technique to make it less intimidating.
- What monitoring is needed? Routine monitoring includes A1C, blood glucose as indicated, kidney function if at risk for dehydration from GI side effects, and clinical monitoring for signs of pancreatitis or gallbladder disease. Report persistent severe abdominal pain, unexplained vomiting, or rapid heart rate immediately.
If any of these answers raise new questions for you—like how to manage nausea or how insurance handles newer drugs—tell me which one and we’ll unpack it together.
Can You Start Mounjaro at 5mg?
Have you wondered whether you can jump straight to 5 mg and skip the starter dose? It’s a common question, especially if you’ve used GLP-1 drugs before and feel ready for a higher dose. The short answer: usually no—clinically we start at 2.5 mg to reduce initial gastrointestinal side effects, but there are exceptions that a clinician might consider.
Here’s why the cautious approach matters. In trials and clinical practice, the initial 2.5 mg weekly dose for the first 4 weeks is intentionally low and is meant to help your body adapt to the medication. Rapid escalation without that acclimation period increases the chance of nausea, vomiting, and early discontinuation—exactly what we want to avoid if the goal is sustained benefit.
- When might a clinician consider starting at 5 mg? If you already tolerate a GLP-1 receptor agonist well (for example, you’ve been on semaglutide or dulaglutide with minimal GI side effects), your clinician might judge that you can begin tirzepatide at 5 mg. This decision weighs your prior drug tolerability, overall health, and the reason for switching.
- What about rapid titration? Some clinicians use faster titration in carefully selected patients, but evidence and product labeling support the slower 4-week steps used in trials. Faster changes may lead to more side effects and potentially interrupt treatment.
- Are there safety or evidence-based reasons to stick to the starter dose? Yes. The SURPASS and SURMOUNT trials used structured titration schedules that began with low doses to optimize tolerability while assessing efficacy across doses. Following evidence-based titration lowers the chance of early drop-out from GI side effects and helps us see the full benefit of therapy.
Let me give you a real-world example: a friend of mine who was already on weekly semaglutide and tolerated it well discussed switching to tirzepatide with their endocrinologist. Because of the prior tolerance, the clinician started at 5 mg and monitored closely—no severe side effects occurred and the patient progressed to higher doses. Contrast that with someone naïve to GLP-1s who started at a higher dose and had to stop within weeks because of severe nausea. So your prior experience matters.
Bottom line: we usually start at 2.5 mg once weekly for 4 weeks, then progress to 5 mg, but starting at 5 mg can be reasonable in selected patients with prior GLP-1 tolerance under a clinician’s supervision. Always discuss your medical history, prior GLP-1 exposure, and preferences with your prescriber so you can choose a plan that maximizes benefit and minimizes side effects.
Can I Stay on 2.5 Mg of Mounjaro?
Have you ever wished you could keep the gentlest setting on a new appliance because it just feels safer? That’s how many people feel about staying on 2.5 mg of Mounjaro — it’s the gentle starter dose and it can be tempting to stay there. Importantly, 2.5 mg is the recommended initiation dose used to help your body adapt to tirzepatide’s effects, especially gastrointestinal side effects like nausea and indigestion.
Clinical guidance and the medication’s prescribing information describe 2.5 mg as a titration dose rather than a long-term therapeutic dose for blood sugar control. In practice, though, there are reasonable situations where a clinician might decide to keep someone at 2.5 mg longer or even indefinitely:
- Tolerability concerns: If severe nausea, vomiting, or other side effects occur, your provider may pause escalation.
- Older or frail patients: When the risks of aggressive glucose lowering or weight loss outweigh benefits, a lower dose may be safer.
- Hypoglycemia risk: If you’re on insulin or sulfonylureas and experience low blood sugars when doses change, staying lower while adjusting other medicines can be wise.
- Patient preference and goals: Some people prioritize fewer side effects over maximal glucose lowering and opt to remain at a lower dose.
Think of it like learning to run after years of sedentary living — some coaches insist you walk a few weeks first. Similarly, many endocrinologists will move up after the standard 4-week starter period if tolerated, but personalization is the rule. Always discuss the plan with your clinician — they’ll balance your blood sugar readings, side effects, other medications, and overall health to decide whether staying at 2.5 mg makes sense for you.
Can You Lose Weight on 2.5 Mg of Mounjaro?
Who wouldn’t want a medication that helps with both blood sugar and the scale? The short answer: yes, you can lose some weight on 2.5 mg, but it’s usually modest. Tirzepatide causes appetite suppression and slower gastric emptying, so even the starter dose can reduce calories eaten and lead to early weight change.
Large clinical programs studying tirzepatide (for example, the SURPASS and SURMOUNT research programs) consistently show a clear dose-response relationship: higher doses produce larger, more consistent weight loss. At 2.5 mg you’re at the bottom of that curve — many people report small initial losses (a few pounds) largely from decreased appetite and changes in how you feel after meals.
Here’s how to frame expectations in everyday terms:
- Early, modest change: You might notice you’re less hungry or that portion sizes shrink naturally — that can translate to a few pounds lost in the first weeks.
- Not the “weight-loss dose”: If significant, sustained weight loss is your primary goal, higher doses studied for weight management produced much larger results.
- Lifestyle still matters: Medication amplifies what you’re already doing. Pairing even 2.5 mg with realistic diet and activity changes will produce better, more durable results than the drug alone.
So if your priority is modest improvement with fewer side effects, 2.5 mg may be satisfying. If you’re chasing substantial weight loss, you and your clinician will likely discuss escalating dose or considering other approved weight-specific therapies. Either way, tracking food, activity, and how you feel helps guide the decision.
When Do You Move From 2.5 Mg to 5 Mg of Mounjaro?
Ready to turn up the dial? The usual roadmap most prescribers follow is straightforward: start at 2.5 mg once weekly for about 4 weeks, then increase to 5 mg if you’re tolerating it. That 4-week starter period is designed to reduce the chance of abrupt gastrointestinal side effects when the medication is first introduced.
But the timing to move from 2.5 mg to 5 mg isn’t only a calendar decision — it’s clinical. Here are the practical criteria clinicians weigh:
- Tolerability: Are nausea, vomiting, or abdominal symptoms controlled or improving? If not, your provider may delay or slow the increase.
- Glycemic response: Have your blood glucose readings moved toward target? If they remain high, escalation is more likely.
- Concomitant medications: If you use insulin or sulfonylureas, your provider may adjust those doses first to avoid hypoglycemia before raising tirzepatide.
- Patient goals and preferences: If you prefer a slower approach, your provider can individualize the titration schedule.
Practical steps when moving up:
- Monitor glucose closely for the first weeks after a dose change and adjust other diabetes medicines as needed.
- Expect temporary GI symptoms — they often improve after several weeks, but if they’re severe, consider holding the increase.
- Watch for red flags such as severe abdominal pain, persistent vomiting, or signs of pancreatitis or gallbladder disease and seek prompt care if they occur.
In short, the label’s standard practice is a 4-week starter at 2.5 mg then increase to 5 mg if tolerated, but we personalize that timeline. Talk with your clinician about what success looks like for you, how to monitor your progress, and when an increase makes sense — that collaborative plan is what keeps you safe and moving toward your health goals.
Can You Take Mounjaro Every 10 Days?
Have you ever wondered whether stretching a weekly medicine out a bit — say, to every 10 days — would make life easier? It’s a tempting thought when schedules get messy or you’re traveling, but with Mounjaro (tirzepatide) there are important reasons we stick to a weekly rhythm.
The short answer: Mounjaro is intended as a once-weekly injection. Its approval, dosing schedules in major trials (for example, the SURPASS program), and the drug’s pharmacology are built around that weekly cadence.
Why once weekly? Tirzepatide has a relatively long half-life (measured in days), which supports sustained activity across the week and allows predictable blood levels when given at regular 7-day intervals. When we drift to every 10 days, blood concentrations can dip lower than expected before the next dose, which may reduce effectiveness for glucose control and weight management and might increase variability in side effects.
That said, life happens. If you accidentally dose a few days late, manufacturers’ guidance and clinical practice typically offer options: for many once-weekly GLP-1/GIP agents, if you miss a scheduled dose you may be advised to take it as soon as you remember within a short window (often a few days) and then resume your usual weekly day after that dose. If too much time has passed, you may be told to skip the missed dose and take the next one on the usual day. Because exact recommendations can differ and labels update, check your prescription information and talk with your clinician before changing timing.
- Example: You normally inject on Mondays but travel and inject on Thursday instead — that’s within a few days and usually acceptable under guidance; stretching to the following Saturday (10 days) isn’t ideal.
- Why it matters: Fluctuations in exposure can affect glucose control, hunger, and side effects like nausea; consistency helps predictability.
In short, try to keep Mounjaro on a weekly schedule. If you’re thinking about routinely dosing every 10 days to simplify life, bring that up with your clinician — they can weigh the pros and cons and help plan around travel, work, or side effects without undermining treatment goals.
What Is the Best Time to Take Mounjaro?
Do mornings work better for you, or is evening the only quiet moment you have? The good news with Mounjaro is there’s flexibility: you can choose a day and time that fits your routine and stick to it.
Key point: Mounjaro is given once weekly and can be injected at any time of day, with or without food. The most important thing is consistency — pick a day/time that you’ll remember week after week.
Here are practical ways people decide:
- Morning routine: If you already have a solid morning ritual (coffee, meds, workout), adding the injection then helps you remember it. Some people prefer mornings because they can monitor how they feel during the day for any side effects like nausea.
- Evening routine: Others choose evenings to avoid daytime nausea at work or because evenings are quieter. If you sleep through mild side effects, evenings may feel more comfortable.
- Same day each week: Whether it’s Tuesday evening or Friday morning, keeping the same weekday helps you track doses and avoids accidental misses.
Clinical trials didn’t show a strong advantage for a specific time of day; instead, they emphasized a weekly schedule. If you’re managing diabetes and timing matters for other medications (insulin, sulfonylureas), coordinate with your provider to minimize hypoglycemia risk when starting or changing Mounjaro.
Finally, think through real-life scenarios: traveling across time zones? Pick a local day/time that’s easy to remember and stick with it. Concerned about nausea? Start with the recommended low starting dose and titrate slowly — timing won’t eliminate nausea but slower increases often make it more tolerable.
Can I Split My Mounjaro Dose?
It’s natural to ask whether splitting a dose might reduce side effects or allow more flexible dosing, but with Mounjaro the answer is generally no.
Tirzepatide is designed and approved as a weekly injectable delivered by a single-dose pen or prefilled injection device in a specific dose. Splitting the dose (for example, taking half one day and half a few days later) isn’t supported by the product design, the clinical data, or safety guidance. Doing so could change how the drug acts in your body and could increase the risk of side effects or reduce effectiveness.
If side effects like nausea or stomach upset are the reason you’re considering splitting doses, there are safer, evidence-based strategies we can use instead:
- Titrate slowly: Clinical protocols usually start at a low dose (for example, 2.5 mg weekly) and gradually increase to higher maintenance doses. Following this step-up schedule reduces GI side effects for many people.
- Supportive measures: Small, frequent meals, staying hydrated, ginger or anti-nausea meds (as advised by your clinician), and avoiding heavy fatty meals around the time you inject can help.
- Timing: Try switching injection time of day if side effects bother you during certain parts of your day (morning vs evening).
- Talk to your clinician: If side effects persist, your provider may adjust the titration schedule or consider alternative therapies.
Practical example: If someone wants to reduce nausea by taking half doses every 3–4 days, that pattern would deviate from the drug’s approved once-weekly dosing and isn’t recommended — instead, we’d work with a healthcare professional to slow the titration or add symptomatic treatments.
Bottom line: don’t split Mounjaro yourself. Let’s focus on approved titration, supportive strategies, and open communication with your care team so you get the benefits while minimizing discomfort.
If any of this raises questions about your own schedule, misses, or side effects, bring specific examples to your clinician — together you can make a practical, safe plan that fits your life.
How Long Can I Take Mounjaro for?
Have you ever wondered if a medication like Mounjaro is meant to be a short sprint or a long-distance run? The short answer is: for many people with type 2 diabetes, Mounjaro (tirzepatide) can be used long term under medical supervision, but how long exactly depends on your goals, benefits, and side effects.
What the evidence and experts say: Clinical trials of tirzepatide (the SURPASS program for diabetes and SURMOUNT for weight loss) studied durations from months up to roughly one to two years and showed sustained benefits in blood sugar control and weight reduction during that time. Endocrinologists often treat Mounjaro as a chronic therapy when it continues to help your A1c, weight, and overall health markers without unacceptable side effects.
Key considerations for long-term use:
- Ongoing benefit: If Mounjaro keeps your A1c in range, reduces diabetes complications risk, or helps with meaningful weight loss, many clinicians will continue it long term.
- Safety monitoring: We watch for gastrointestinal tolerance, gallbladder issues, pancreatitis symptoms, kidney function, and very rarely thyroid concerns (rodent studies showed thyroid C‑cell tumors; human risk appears different but it’s monitored). Regular labs and checkups are important.
- Medication interactions: If you’re on insulin or sulfonylureas, long-term use requires careful dose adjustments to avoid hypoglycemia.
- Data limits: While trials show benefits up to around 1–2 years, truly long-term (many years) safety and outcomes are still being studied; that’s why follow-up with your clinician matters.
Real-world example: Imagine Sarah, who started Mounjaro for type 2 diabetes. After six months her A1c dropped, she lost weight, and her energy improved. Together with her endocrinologist she continued therapy, had regular labs, and tapered her insulin—turning a short-term experiment into a sustainable management plan.
So, can you take it indefinitely? Possibly, yes—if it’s effective and well tolerated—but it’s not a “set and forget” medicine. We should review benefits, side effects, and alternatives periodically and make decisions together.
What Happens If You Use Too Much Mounjaro?
Accidentally taking an extra injection or taking more than your prescribed weekly dose can be scary. What should you expect, and how is it handled?
Immediate effects of an overdose:
- Intense gastrointestinal symptoms: Nausea, vomiting, severe diarrhea, and abdominal pain are the most common reactions because tirzepatide strongly affects appetite and GI motility.
- Dehydration and electrolyte loss: Repeated vomiting or diarrhea can lead to dehydration, dizziness, low blood pressure, and in severe cases, acute kidney injury.
- Hypoglycemia risk: If you’re also taking insulin or sulfonylureas, an extra dose can precipitate low blood sugar—symptoms range from shakiness and sweating to confusion or loss of consciousness.
- Pancreatitis and gallbladder issues: Although rare, severe abdominal pain, persistent vomiting, or fever could signal pancreatitis or gallbladder disease; these require urgent evaluation.
Why an extra dose can last longer than you expect: Mounjaro is given once weekly and has a long duration of action, so an extra dose can prolong side effects for days. There’s no rapid “antidote” to clear the drug—care is mainly supportive.
What to do if it happens:
- Call your healthcare provider or local poison control right away and explain the dose and timing.
- If you’re dizzy, very weak, vomiting, or showing signs of severe hypoglycemia (confusion, fainting), seek emergency care.
- Bring a list of other diabetes medicines—if you use insulin or sulfonylureas, clinicians will be especially careful to check blood sugars and may give intravenous fluids or electrolytes.
- Treat symptoms: anti-nausea medication, IV fluids for dehydration, and close glucose monitoring are common approaches.
Prevention tips: Use weekly reminders, keep pens in a consistent place, and double-check labels before injection. If you travel or shift schedules, plan doses with your provider to avoid accidental extra injections.
Can I Switch From Wegovy to Mounjaro?
Thinking of switching because you’ve heard tirzepatide may produce greater weight loss or you want to try a different approach? It’s a reasonable question—and one many people ask in clinic.
How these drugs differ: Wegovy is semaglutide, a GLP‑1 receptor agonist approved for chronic weight management. Mounjaro is tirzepatide, a dual GIP/GLP‑1 receptor agonist approved for type 2 diabetes (and a tirzepatide formulation later gained approval for weight management under a different brand name). The dual action often leads to different and sometimes larger effects on appetite and weight.
Is switching possible and safe? Yes—with planning. There’s no one-size-fits-all script, but common principles include:
- Talk to your clinician first: We’ll review why you want to switch (effectiveness, side effects, access), check other medications, and plan the transition.
- Expect overlapping side effects: Because both affect the same GI pathways, switching can temporarily increase nausea, vomiting, or other digestive symptoms. Starting tirzepatide at a low dose and titrating helps reduce this.
- Typical practical approach: Many clinicians stop semaglutide and begin tirzepatide at the recommended starting dose (commonly 2.5 mg weekly) at the next scheduled injection time or shortly after; others may allow a brief washout if side effects have been severe. Individual practice varies, so individualized plans matter.
- Adjust other diabetes drugs: If you’re on insulin or sulfonylureas, anticipate dose reductions to prevent hypoglycemia when tirzepatide is started.
What evidence guides this? Direct head-to-head long-term trials between semaglutide 2.4 mg (Wegovy dose) and tirzepatide are limited, but clinical trial programs show tirzepatide can produce substantial weight loss and glycemic improvements. That’s why some people switch. Still, personal tolerance, prior response to GLP‑1 therapy, and insurance coverage often determine the decision.
A practical vignette: Raj was on Wegovy and lost 10% of his body weight but plateaued. After discussing options with his endocrinologist, he stopped semaglutide and started tirzepatide at a low dose. He experienced a week of increased nausea but then improved appetite control and additional weight loss when titrated slowly. His provider monitored blood sugars and adjusted other meds.
In short: you can often switch from Wegovy to Mounjaro, but do it with a plan—start low, go slow, monitor, and stay in close contact with your care team so we can manage side effects and medication interactions together.