Have you ever heard someone say “semaglutide” and “Ozempic” like they’re interchangeable and wondered if that’s really true? In short, semaglutide is the active drug molecule, while Ozempic is a brand name product that contains semaglutide formulated and dosed specifically for treating type 2 diabetes. Think of semaglutide like flour and Ozempic like a particular brand of cake mix: same core ingredient, different recipe, packaging, and instructions.
Clinically, semaglutide has been studied in large trial programs — the SUSTAIN series for diabetes and the STEP series for weight management — showing meaningful improvements in blood sugar control and body weight. That’s why you’ll see the same molecule appear across different products with different names and purposes: Ozempic for weekly injectable diabetes treatment, Wegovy (the brand name for a higher-dose injectable semaglutide) for chronic weight management, and Rybelsus as an oral form of semaglutide for diabetes. Those differences matter when we talk about dosing, device delivery, and what your prescriber intends you to use.
So when you and your clinician discuss “semaglutide,” it helps to clarify: are we talking about the molecule in any form, or the specific branded product and dose you’ll be prescribed?
Key Takeaways
- Active ingredient vs. brand: Semaglutide is the medication; Ozempic is a branded semaglutide product for type 2 diabetes.
- Different indications and dosing: Brands that contain semaglutide (Ozempic, Wegovy, Rybelsus) are approved for different conditions and use different doses and delivery methods.
- Formulation and device matter: Pen design, dose escalation schedules, and concentration differ between products — you can’t automatically substitute one for another without medical guidance.
- Safety and sourcing are important: Compounded or off-label semaglutide preparations carry extra risks; consult reliable sources and your provider before using alternatives.
What Is the Difference Between Semaglutide and Ozempic?
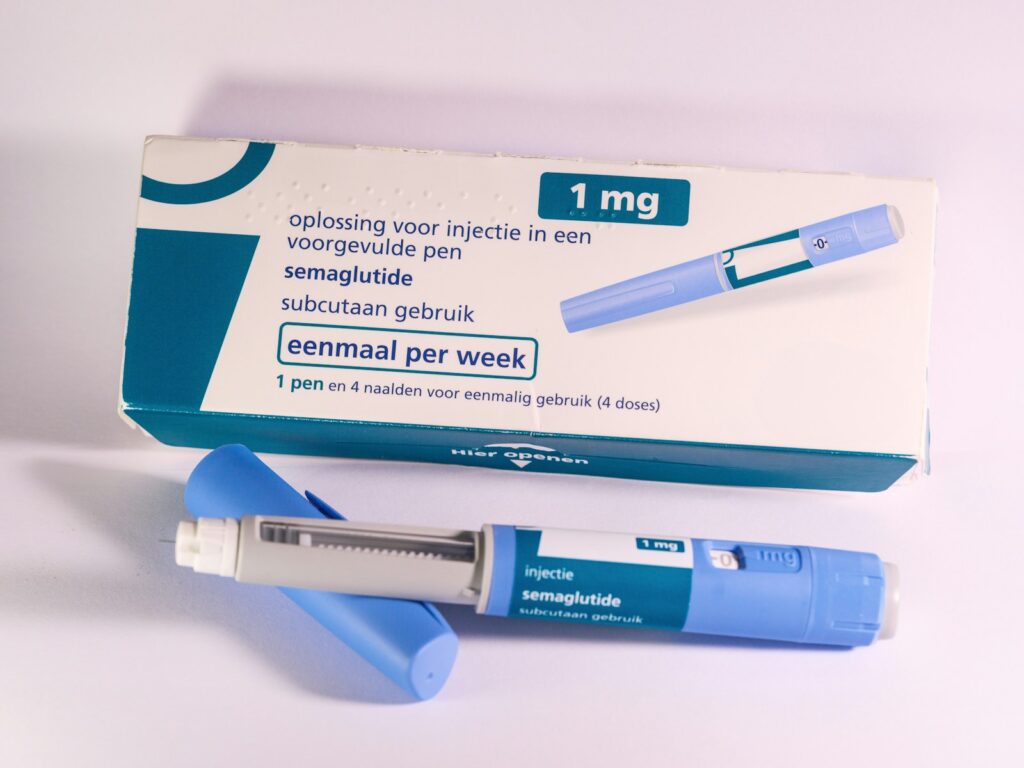
Curious about the practical differences? Let’s walk through the key areas where they diverge — dosing, approvals, formulation, and real-world considerations — while keeping things grounded in everyday choices you might make.
1) The molecule vs. the product: Semaglutide is the generic name for the GLP-1 receptor agonist molecule. Ozempic is Novo Nordisk’s weekly injectable product that contains semaglutide in a specific concentration with a pen device and an approved dosing schedule. This distinction is similar to aspirin vs. a branded aspirin tablet: the active chemistry can be the same, but how it’s packaged and prescribed can differ.
2) Indications and dose differences: Ozempic is FDA-approved for treating type 2 diabetes at particular weekly doses (starting low and titrating up). For chronic weight management, manufacturers developed a higher-dose semaglutide brand (Wegovy) that uses a different titration schedule and a higher target dose. The difference in approved doses is why your prescriber won’t simply swap a diabetes pen for a weight-loss pen without a plan. Large clinical trials — SUSTAIN for diabetes and STEP for obesity — demonstrated efficacy at the dosing ranges used in those approvals, which is why the labels differ.
3) Formulation, devices, and administration: Each brand provides specific pens or tablets, instructions for home use, and dose escalation schedules designed to reduce gastrointestinal side effects and ensure safety. For example, weekly injectable pens for Ozempic are calibrated differently than the pen used for Wegovy, even though both deliver semaglutide. There’s also an oral semaglutide product (Rybelsus) that shows how the same molecule can require different formulations to work as intended.
4) Safety, monitoring, and side effects: Common side effects of GLP-1 receptor agonists include nausea, vomiting, and sometimes constipation or diarrhea; these effects are typically dose-related and often diminish with gradual dose increases. There are rarer but serious concerns that clinicians monitor for, which is why you should follow your prescriber’s plan and report symptoms promptly. Regulatory bodies and medical boards have issued guidance and warnings about appropriate prescribing and potential risks — you can read one such press release on concerns with semaglutide and other GLP‑1 receptor agonists if you want the regulatory perspective.
5) Compounded or off-label products: You may see clinics or pharmacies offering compounded semaglutide or other nonbranded preparations at lower prices or different doses. That can be tempting, but it introduces quality and safety questions because compounding bypasses standard manufacturing controls. Health reporting has covered how compounded semaglutide differs from Ozempic and why experts urge caution — for a balanced primer, consider this overview of compounded semaglutide versus Ozempic.
6) Cost, access, and real-world choices: Insurance coverage often follows FDA-approved indications, so cost and copays can vary widely between Ozempic, Wegovy, and other branded or compounded options. If you’re shopping for a pharmacy or trying to compare patient experiences, it can help to consult trusted resources — for example, many people look up pharmacies like Coreage Rx for fulfillment options, or read community feedback in patient Reviews to learn how others navigated coverage and side effects.
At the end of the day, we can summarize this way: semaglutide is the chemical foundation; Ozempic is one clinically tested, regulated, and marketed way to deliver that medicine for people with type 2 diabetes. If you and your clinician are discussing semaglutide therapy, the important questions to ask are: Which brand and dose is intended? What is the approved indication? How will dosing be titrated, and how will we monitor for side effects?
Have you or someone you know tried semaglutide in any form? What was the experience like — the good and the surprising? Sharing those stories with your care team helps tailor treatment to the life you actually live, and we’re here to help you translate what the labels and trials mean for your daily routine.
What Is Semaglutide?
Have you ever wondered what people mean when they say “semaglutide” without naming a brand? At its core, semaglutide is the generic name for a medication class called GLP-1 receptor agonists, a type of drug that mimics a natural gut hormone (glucagon-like peptide-1) to help regulate blood sugar and appetite. Clinically, that translates into two everyday effects: better blood sugar control and reduced hunger or food intake — which is why it’s become central in both diabetes care and weight management conversations.
From a scientific angle, semaglutide works by slowing gastric emptying, increasing insulin secretion when glucose is high, and decreasing glucagon release. Its pharmacology gives it a relatively long duration of action — the injected form is designed for once-weekly dosing, because the molecule’s half-life is on the order of days. Large clinical programs have supported its use: the SUSTAIN trials established benefits for glycemic control and heart-related outcomes, while the STEP trials showed substantial weight loss when used at higher doses.
Experts — from endocrinologists to primary care clinicians — will tell you that semaglutide is powerful but not magic: side effects like nausea and other gastrointestinal symptoms are common early on, and long-term safety monitoring continues. If you’re weighing options for type 2 diabetes or chronic weight management, it helps to think of semaglutide as the active ingredient that can appear in different products and doses, rather than a single branded experience. For a consumer-friendly comparison of semaglutide and branded products, this overview explains the distinction well: is semaglutide the same as Ozempic?
What Is Ozempic?
Curious why you hear “Ozempic” in conversations about diabetes or weight loss so often? Ozempic is one brand of semaglutide made by Novo Nordisk, formulated and approved primarily for the treatment of type 2 diabetes as a once-weekly injectable. Think of Ozempic as one specific vehicle for delivering semaglutide — it contains the same active molecule, but its approved dosing, labeling, and manufacturer support are distinct from other semaglutide products.
In practice, clinicians choose Ozempic when they want the proven diabetes benefits from the SUSTAIN clinical program, including improved A1C and, in some studies, a reduced risk of major adverse cardiovascular events for people at high cardiovascular risk. The typical Ozempic dosing schedule starts with lower doses to reduce GI side effects and titrates upward based on response and tolerance. Patients often report meaningful reductions in appetite and weight alongside better glucose numbers — which is part of why the brand name became so prominent outside clinics.
But here’s a real-world nuance we share with patients: because semaglutide appears in different products, people sometimes assume Ozempic and other brands are interchangeable in dose and purpose. That’s not always true. Different formulations and labeled indications — for example, a product marketed specifically for weight management — can mean different target doses and treatment plans. If you’re exploring options, it helps to compare not just the active ingredient but the specific brand’s approved use, dose, and insurance coverage; a consumer-facing comparison that dives into these distinctions is useful: semaglutide vs Ozempic explained
Other Brand Names for Semaglutide
What if I told you semaglutide shows up under several different labels? That’s true — and it’s the main reason confusion spreads quickly. Here are the names you’ll most often encounter and what they mean for you:
- Ozempic — the once-weekly injectable approved primarily for type 2 diabetes; known from the SUSTAIN program for glycemic control and some cardio benefits.
- Wegovy — the brand of semaglutide specifically approved at a higher dose (2.4 mg weekly) for chronic weight management; the STEP trials are the big clinical program showing impressive average weight loss compared with placebo.
- Rybelsus — an oral form of semaglutide taken daily; it offers an alternative for people who prefer pills over injections but uses lower dosages and has different absorption considerations.
- Generic or compounded semaglutide — in some markets you’ll see non-branded versions or compounding pharmacy products; availability, regulation, and quality can vary, so clinicians urge caution.
How do clinicians decide among these? It comes down to indication, dose, formulation, and the patient’s goals. If your primary goal is blood sugar control and you have heart disease risk, Ozempic may fit. If the goal is focused, intensive weight loss and you meet criteria, Wegovy’s labeled dose is used. If you prefer an oral medication, Rybelsus offers semaglutide in pill form. We also compare semaglutide to other diabetes agents when weighing options — for instance, SGLT2 inhibitors like empagliflozin (Jardiance) also cause weight loss and bring cardiovascular benefits in some patients; if you want a deeper dive into weight effects of other diabetes meds, see: Does Jardiance Cause Weight Loss.
Want a quick takeaway? Semaglutide is the molecule; Ozempic is one brand that contains it. The brand matters because of dose, approval, and how it’s prescribed. When we talk to patients, we try to connect scientific facts to everyday choices: how you feel after meals, how a prescription fits your schedule, and how insurance or out-of-pocket cost shapes what’s realistic. If you’re considering semaglutide in any form, let’s talk about your goals, tolerance for side effects, and the practical details that will make a medication fit into your life.
Rybelsus
Have you ever wished a pill could do what injections do? That’s where Rybelsus often surprises people. Rybelsus is an oral formulation of semaglutide approved for type 2 diabetes management; because the pill is formulated to survive the stomach and be absorbed, its dosing and practical use differ significantly from injectable versions. For many patients, the ease of taking a daily tablet instead of learning injection technique feels like a game-changer, but there are important trade-offs to understand.
Functionally, Rybelsus works the same way as other semaglutide products — it’s a GLP-1 receptor agonist that helps increase insulin secretion in response to glucose, reduces inappropriate glucagon release, slows gastric emptying, and reduces appetite — but the oral route requires lower, carefully calibrated doses (commonly 3 mg, 7 mg, 14 mg) and specific administration rules: take it on an empty stomach with a small amount of water and wait about 30 minutes before eating or taking other medications. That routine can feel fussy at first, but many people quickly fold it into their morning ritual.
Clinical trials and real-world reports show Rybelsus meaningfully improves HbA1c and supports modest weight loss in people with type 2 diabetes, though the weight-loss effect is generally smaller than the higher-dose injectable formulation used for obesity. If you’re weighing options, a good way to think about it is: Rybelsus prioritizes oral convenience for diabetes control, while injectable semaglutide products prioritize higher, weight-loss-focused exposures. For a clear, clinician-oriented comparison of semaglutide formulations and how they differ in practice, this comparison is helpful: differences between semaglutide formulations.
- Who might choose Rybelsus: people uncomfortable with injections who need glucose-lowering therapy and are able to follow the fasting/administration routine.
- Common side effects: nausea, early satiety, diarrhea, occasional vomiting — typically improving over weeks with dose titration.
- Important notes: oral bioavailability is low, which is why doses differ from injectables; it’s not approved specifically for chronic weight management at the higher doses used in obesity trials.
Have you ever had to switch from a pill to an injection? Many patients report that once they understand the benefits and the dosing schedule, they feel more confident selecting the option that fits their life.
Wegovy
Looking for a medication prescribed primarily for weight loss rather than diabetes control? Wegovy might be what you’ve heard about. Wegovy is semaglutide at a higher weekly dose (2.4 mg) delivered by injection and approved specifically for chronic weight management in adults with obesity or overweight with at least one weight-related condition. It’s designed and studied to help people lose substantial weight when combined with lifestyle interventions.
Why is Wegovy different from other semaglutide products? It’s largely dose and indication. The STEP program of trials — a series of randomized controlled studies — demonstrated average weight losses often in the double-digit percentage range (for example, many participants lost 10–15% of baseline body weight at 68 weeks in major trials), outcomes that are clinically meaningful for cardiovascular risk and quality of life. Those results contrast with the more modest weight reductions seen with lower-dose semaglutide products used primarily for diabetes.
As you consider expectations, remember that experts emphasize combining medication with nutrition, activity, and behavioral support. If you’ve read headlines about rapid weight loss on drugs like Ozempic or Wegovy, you’ll find balanced clinical guidance here: experts’ recommendations on semaglutide and weight loss. That piece captures the common clinical counsel: these medicines can be powerful tools, but they work best with ongoing lifestyle change and medical follow-up.
- Who might use Wegovy: adults meeting BMI and comorbidity criteria who want a medically supervised path for significant weight loss.
- Expected effects: substantial weight reduction over months, improved metabolic markers in many patients, and often improvements in blood pressure and quality of life.
- Side effects and considerations: gastrointestinal symptoms are common during titration; rare but serious risks (e.g., pancreatitis, potential thyroid C-cell tumor concerns in rodents) are discussed with your clinician before starting.
If you imagine long-term weight management as a journey rather than a quick fix, Wegovy is one of the more effective medical tools we have today — yet it’s a journey best taken with a healthcare team and realistic expectations.
Uses and Indications
So, is semaglutide the same as Ozempic? The short answer is nuanced: semaglutide is the active drug molecule; Ozempic, Wegovy, and Rybelsus are different brand formulations and doses of that molecule designed for particular indications and routes of administration. Understanding uses and indications helps you and your clinician choose the right brand, dose, and monitoring plan.
- Ozempic: injectable semaglutide approved for type 2 diabetes to improve glycemic control. Typical weekly starting doses are low and titrated (0.25 mg for initiation, then commonly 0.5–1 mg weekly; higher doses may be used in some regions).
- Wegovy: injectable semaglutide at a fixed higher dose (2.4 mg weekly) approved specifically for chronic weight management in patients who meet BMI/health criteria.
- Rybelsus: oral semaglutide tablets (daily) approved for type 2 diabetes; dosing and administration differ (fasting requirement) and it’s not the weight-management dose used in Wegovy.
- Off-label and overlapping uses: clinicians sometimes use Ozempic for off-label weight loss and may prescribe different semaglutide forms based on patient need, insurance coverage, and tolerability — but official approvals guide insurance and standardized care.
Mechanism-wise, these products share actions: enhanced glucose-dependent insulin secretion, reduced glucagon, slowed gastric emptying, and appetite suppression via central nervous system pathways. That shared biology explains why similar side effects and benefits appear across formulations, but dose and route shape the intensity of effects — for example, the higher weekly dose used in Wegovy produces larger effects on appetite and weight than the lower doses used in diabetes-focused products.
When deciding between products, clinicians look at several real-world factors: what condition we’re treating (diabetes vs. obesity), how comfortable you are with injections, lifestyle fit (can you take a pill on an empty stomach every morning?), insurance coverage and cost, and medical history (personal or family history of medullary thyroid carcinoma or MEN2 is a contraindication). We also watch for interactions: combining GLP-1 receptor agonists with insulin or sulfonylureas raises hypoglycemia risk, so glucose-monitoring and potential dose adjustments matter.
Thinking practically: imagine two patients. One has newly diagnosed type 2 diabetes, strong needle phobia, and reliable morning routines — Rybelsus may be an appealing first step. Another has obesity with failed lifestyle-only attempts and desires substantial weight loss; Wegovy with structured lifestyle support may be recommended. Both choices require dose titration, follow-up, and honest conversations about side effects and expectations.
Have other questions about how these medicines fit into daily life, safety monitoring, or cost and access? Let’s talk through the specifics — we can map options to your health goals and experiences so you know what to expect.
Off-Label Semaglutide Uses
Curious how a diabetes medication ended up in conversations about weight loss, liver disease, and appetite control? You’re not alone — semaglutide started as a treatment for type 2 diabetes but its effects on appetite and metabolism have led clinicians and researchers to try it in a range of other conditions.
What clinicians are using it for off-label? The most common off-label use is for weight management: many people prescribed semaglutide for diabetes notice substantial weight loss, and some providers prescribe it specifically to help with obesity even if the particular formulation (like Ozempic) isn’t formally approved for that indication. Other off-label areas under investigation or in limited clinical use include nonalcoholic steatohepatitis (NASH), polycystic ovary syndrome (PCOS) where weight and insulin resistance play roles, and binge eating disorder, where reductions in appetite and cravings may help. Smaller studies and case series have also explored effects on glycemic variability, prediabetes, and even cardiovascular risk markers.
If you want a concise comparison that helps explain why different semaglutide products get used in different ways, this comparison of semaglutide and Ozempic is a helpful primer that clinicians sometimes share with patients.
What does the evidence say? Large randomized trials designed specifically for weight management (the STEP program) showed that semaglutide at higher weekly doses produced meaningful and sustained weight loss — often in the ballpark of double-digit percentage reductions from baseline over months when combined with lifestyle support. For conditions like NASH and PCOS the evidence is smaller but promising: some trials show improvements in liver enzymes and metabolic markers or in menstrual regularity and ovulation when weight loss occurs. Experts caution, however, that off-label use lacks the same depth of regulatory scrutiny and long-term safety data that we have for on-label indications.
Practical perspective: Think of semaglutide as a powerful tool that can change appetite, eating behavior, and metabolic set points — and like any powerful tool, it works best when used thoughtfully. Weighing potential benefits against unknowns (especially for long-term use in conditions other than diabetes or FDA-approved obesity treatments) is something to discuss with your clinician.
Is Compounded Semaglutide or Ozempic FDA Approved for Weight Loss?
Have you seen compounded semaglutide advertised as a “cheap” alternative, or wondered whether Ozempic is officially a weight-loss drug? Let’s unpack that in plain terms.
Ozempic is a brand-name medication approved by the FDA for type 2 diabetes. It contains semaglutide as the active ingredient and is prescribed to improve glycemic control; you can read the manufacturer’s explanation of what Ozempic is and how it’s used on the Ozempic site. Because weight loss is a common and often desirable side effect, many clinicians and patients noticed its potential for obesity treatment.
But FDA approval for weight loss is product-specific. In the United States, a semaglutide product marketed as Wegovy received FDA approval specifically for chronic weight management at a higher dose than Ozempic typically uses for diabetes. That means not every semaglutide formulation is FDA-approved for obesity; Ozempic, while effective at producing weight loss in many patients, is not the obesity-labeled product.
What about compounded semaglutide? Compounded versions are made by compounding pharmacies rather than the drug manufacturer and are sometimes promoted as lower-cost or as custom-dosed alternatives. These compounded preparations are not FDA-approved as weight-loss drugs. That raises several concerns:
- Quality and consistency: Potency and sterility can vary more with compounding; you don’t get the same manufacturing oversight as with an FDA-approved product.
- Dosing and evidence: Compounded versions often lack the controlled, clinical-trial–based dosing schedules that established approvals rely on.
- Liability and regulation: If adverse events occur, tracking and regulatory recourse are more complicated than with a branded, approved drug.
In short, if weight management is your goal, we can look at proven, FDA-approved options and structured programs first. If someone offers compounded semaglutide, ask for clear documentation on sterility testing, dosing accuracy, and why they believe it’s safer or more effective than an approved alternative — and discuss it with your provider before starting it.
Safety and Side Effects
What should you watch for if you or someone you care about starts semaglutide? Safety isn’t just a list of rare words — it’s about anticipating everyday experiences and knowing when to call your clinician.
Common, usually manageable side effects tend to be gastrointestinal and appear early in treatment or during dose increases. Expect symptoms like:
- Nausea and vomiting: Often transient; many people report these on dose titration and see improvement over weeks.
- Diarrhea or constipation: Changes in bowel habits are frequent and can be managed with dietary tweaks and hydration.
- Decreased appetite: Part of why semaglutide helps with weight loss but can be uncomfortable at the start.
Clinical trials such as the STEP program documented these patterns: GI side effects were the most commonly reported, and they were a frequent reason for early discontinuation in some participants. Knowing that these are expected and often time-limited can be reassuring, and simple measures — smaller meals, slower titration, or temporary antiemetics — sometimes help.
Less common but serious risks: There are a few important serious events you should be aware of:
- Pancreatitis: Acute pancreatitis has been reported with GLP-1 receptor agonists. Seek immediate care for severe, persistent abdominal pain.
- Gallbladder disease: Rapid weight loss can increase gallstone risk; watch for right upper quadrant pain, fever, or jaundice.
- Kidney issues: Dehydration from vomiting or diarrhea can worsen kidney function in susceptible people.
- Thyroid C-cell tumors: In rodent studies, semaglutide was associated with C-cell tumors. While the human relevance is unclear, products carry contraindications for people with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 (MEN2).
- Hypoglycemia: When combined with insulin or insulin secretagogues (like sulfonylureas), the risk of low blood sugar increases — dose adjustments and close monitoring are essential.
How to reduce risk in everyday life:
- Start low and go slow: gradual dose escalation reduces GI side effects for many people.
- Stay hydrated and eat small, frequent meals when nausea is present.
- Tell your clinician about all medications — especially insulin or sulfonylureas — so they can adjust doses.
- Report severe abdominal pain, persistent vomiting, signs of gallbladder disease, or symptoms of hypoglycemia immediately.
- Avoid pregnancy: semaglutide is not recommended during pregnancy and effective contraception is advised while using it and for a period after stopping as directed by your clinician.
Balancing benefits and concerns: Many people experience meaningful improvements in weight, glycemic control, and even quality of life, but these gains come with trade-offs that are highly individual. Weighing them means asking: How much does this symptom profile affect your daily life? Are there comorbid conditions that increase risk? What monitoring and follow-up can your care team provide?
If you’re considering semaglutide or currently taking it, let’s keep a running conversation with your provider about goals, side effects, and the safest path forward — that shared decision-making is how we get the most benefit with the least harm.
Important Safety Information
Have you ever wondered what to watch for when a medication becomes a household name? When we talk about Ozempic® (a brand of the GLP‑1 drug semaglutide), safety is the conversation we want to have first — because what looks like a simple injection can affect many parts of your body. Clinicians and patients alike have learned that while these medicines can be powerful, they also come with specific risks and precautions that matter in everyday life: from the way your morning coffee sits in your stomach to how your eyes respond to changes in blood sugar.
Quick reality check: Ozempic® contains semaglutide, but not every semaglutide product is the same in dose or indication — and GLP‑1 drugs differ in profiles and uses. If you’re comparing options or trying to understand how Ozempic® stacks up against other GLP‑1s, take a look at a clear comparison of GLP‑1 drugs to see differences in dosing, indications, and side‑effect patterns: compare GLP‑1 medications and formulations.
- Gastrointestinal effects are common. Nausea, vomiting, diarrhea, and constipation are the most frequent complaints; they often occur when treatment starts or the dose increases. Many patients adapt over weeks, but some need slower dose titration or supportive care.
- Pancreatitis is a serious but uncommon risk. If you develop persistent severe abdominal pain (sometimes with vomiting), providers are trained to evaluate for pancreatitis and stop the medication if it’s suspected.
- Potential kidney effects. Dehydration from prolonged vomiting or diarrhea can worsen kidney function. People with existing kidney disease should be monitored closely.
- Eye complications in people with long‑standing diabetes. Clinical trials, including SUSTAIN‑6, noted an increase in diabetic retinopathy complications in some patients treated with semaglutide — especially when blood glucose falls quickly. That’s why eye exams and careful glycemic control matter.
- Hypoglycemia risk when combined with other diabetes medicines. If you’re also on insulin or sulfonylureas, your risk of low blood sugar goes up — and doses of those drugs may need to be adjusted.
Those points capture the landscape, but the nuance comes from conversation: your age, other medications, kidney function, pregnancy plans, and whether you have a family history of certain cancers all change the safety equation. Let’s walk through the specific “most important” facts next so you know exactly what to bring up at your next appointment.
What Is the Most Important Information I Should Know About Ozempic®?
What’s the single most important takeaway? It’s a blend of two ideas: the specific, serious risks you should never ignore, and the everyday practicalities that determine whether Ozempic® is right for you. Here’s what clinicians emphasize when they talk to patients for the first time.
- Thyroid C‑cell tumor risk (rodent studies) and MTC concern. In rodent studies, semaglutide caused thyroid C‑cell tumors. It’s unknown if this risk applies to humans, but because of those findings, Ozempic® is not recommended for people with a personal or family history of medullary thyroid carcinoma (MTC) or for those with multiple endocrine neoplasia syndrome type 2 (MEN2).
- Watch for signs of pancreatitis. Sudden, severe abdominal pain — sometimes radiating to the back and accompanied by nausea/vomiting — should prompt immediate medical attention and discontinuation of the drug until pancreatitis is ruled out.
- Risk of severe hypoglycemia with insulin or sulfonylureas. If you’re taking agents that lower glucose by increasing insulin (like insulin itself or sulfonylureas), you and your clinician should anticipate dose changes and teach you how to recognize and treat low blood sugar.
- Renal monitoring during episodes of significant dehydration. Prolonged vomiting or diarrhea can lead to dehydration and acute kidney injury — a practical day‑to‑day reason to report prolonged GI symptoms early.
- Possible worsening of diabetic retinopathy. Rapid improvements in blood sugar control have been associated with transient worsening of retinopathy in some trials; if you have background diabetic eye disease, regular retinal checks are important.
- Not for type 1 diabetes or diabetic ketoacidosis. Ozempic® is indicated for type 2 diabetes; it is not a substitute for insulin in type 1 diabetes or for treating diabetic ketoacidosis.
- Pregnancy and breastfeeding considerations. If you are pregnant, planning pregnancy, or breastfeeding, discuss alternatives — many clinicians advise stopping semaglutide because the safety profile in pregnancy and lactation is not well established.
An endocrinologist I spoke with often frames it this way: “These drugs can transform the lives of patients with type 2 diabetes, but they need the same respect we give to insulin — dosing, monitoring, and follow‑up.” That’s why the “most important” information is as much about ongoing communication with your care team as it is about the initial prescription.
Do Not Use Ozempic® If:
Could this medication be unsafe for you? Here are clear situations where Ozempic® is contraindicated or strongly discouraged — think of these as red flags that should stop you from starting treatment until you’ve discussed alternatives with your clinician.
- You or a close blood relative has had medullary thyroid carcinoma (MTC). Because of the rodent findings, a personal or family history of MTC is a firm contraindication.
- You have multiple endocrine neoplasia syndrome type 2 (MEN2). MEN2 confers high risk for MTC; Ozempic® should not be used.
- You’ve had a severe allergic reaction to semaglutide or any product ingredient. Hives, swelling, difficulty breathing, or other signs of anaphylaxis mean this medication is not appropriate.
- You have type 1 diabetes or are treating diabetic ketoacidosis. Ozempic® is not indicated in these situations and should not replace insulin therapy.
- You are a child or adolescent under the approved age for this formulation. Ozempic® is approved for adults with type 2 diabetes; pediatric use depends on specific product approvals and should be handled by a specialist.
If any of these apply to you, we should pause and talk through alternatives and next steps. Even when none of these contraindications are present, it’s wise to discuss baseline labs, eye exams, and an individualized plan for dose titration so you and your clinician can spot problems early and keep you safe while benefiting from treatment.
Before Using Ozempic®, Tell Your Health Care Provider If You Have Any Other Medical Conditions, Including If You:
Have you ever felt unsure which details matter when starting a new medication? When it comes to Ozempic® (semaglutide), sharing your full medical story with your health care provider can change outcomes — sometimes dramatically. Here are key conditions and situations you should definitely mention.
- Have a personal or family history of medullary thyroid carcinoma (MTC) or MEN2 — Clinical guidance and the drug label note a potential risk for thyroid C‑cell tumors in rodents; because of that, Ozempic® is not recommended for people with these histories.
- A history of pancreatitis — Although direct causation is debated, pancreatitis has been reported with GLP‑1 receptor agonists. Your provider will weigh risks and monitor you closely.
- Diabetic retinopathy or advanced eye disease — In the SUSTAIN‑6 trial, semaglutide was associated with a higher rate of worsening diabetic retinopathy events in patients with pre‑existing retinopathy, so eye disease history matters.
- Severe kidney disease or recent kidney injury — Nausea, vomiting, or dehydration from side effects can worsen kidney function; your clinician may want to monitor labs or adjust other medicines.
- Severe gastrointestinal disease, including gastroparesis — Because semaglutide slows gastric emptying, symptoms like chronic nausea or delayed stomach emptying can be exacerbated.
- History of gallbladder disease or gallstones — Rapid weight loss and GLP‑1 therapies have been associated with gallbladder issues in some patients.
- Pregnancy, planning pregnancy, or breastfeeding — Safety in pregnancy and lactation is not established; your provider will discuss alternatives.
- A history of severe allergic reactions — If you’ve had hypersensitivity to semaglutide or any product components, Ozempic® is not appropriate.
- Taking other medications that affect blood glucose — If you use insulin or sulfonylureas, there’s an increased risk of hypoglycemia when combined with Ozempic®; your dosing plan may need adjustment.
Think of your medical history as a puzzle — each piece helps your clinician decide whether the benefits of Ozempic® outweigh the risks. For example, I’ve seen patients with long‑standing retinopathy get an extra eye exam scheduled before and after starting semaglutide so small changes are caught early. That simple extra step often prevents surprises.
When in doubt, tell your provider: even what seems minor — like a bout of unexplained abdominal pain last year or a close relative with a rare thyroid tumor — can alter the decision. We tend to underestimate how connected conditions are; sharing openly gives you safer, more tailored care.
What Are the Possible Side Effects of Ozempic®?
Wondering what to expect and how to tell normal adjustment from something that needs attention? Here’s a practical breakdown based on trial data, clinical experience, and safety reports.
- Common, usually temporary gastrointestinal effects: nausea, vomiting, diarrhea, constipation, abdominal pain, and decreased appetite. In clinical trials these were the most frequent complaints — nausea often appears early and fades with time as your body adjusts.
- Weight loss and reduced appetite: Many people consider this a benefit, but it can be excessive for some and should be monitored, especially if you’re frail or have eating disorders.
- Hypoglycemia (low blood sugar): More likely when Ozempic® is used with insulin or insulin secretagogues like sulfonylureas. Expect your provider to discuss glucose monitoring and possible dose reductions of other diabetes meds.
- Pancreatitis: Though not definitively proven as causal, cases have been reported. Seek immediate care for severe persistent abdominal pain with or without vomiting.
- Gallbladder disease: Rapid weight change and GLP‑1 therapies have been linked to gallstones and cholecystitis in some patients.
- Kidney injury: Dehydration from prolonged vomiting or diarrhea can worsen kidney function — especially in those with pre‑existing kidney disease.
- Injection site reactions and allergic reactions: Some people get redness or itching at injections; rare serious hypersensitivity reactions can occur.
- Potential thyroid C‑cell tumor risk: Observed in rodent studies; relevance to humans is unclear but the medication is contraindicated for people with MTC or MEN2.
- Worsening diabetic retinopathy: A signal seen in the SUSTAIN‑6 cardiovascular outcomes trial. If you have diabetic eye disease, plan closer ophthalmology follow‑up.
How you manage side effects matters: start low and titrate as recommended, eat small, bland meals when you feel nauseated, stay hydrated, and communicate early with your care team. I once had a friend start semaglutide and feel queasy the first two weeks; with guidance she adjusted meal timing and the nausea largely resolved. But for another patient, persistent vomiting led to a clinic visit and temporary medication hold — timely action made all the difference.
Ask yourself: Is the discomfort worth the benefit? For many people with type 2 diabetes, Ozempic® improves blood sugar control and has cardiovascular benefits, but we balance those gains against side effects and personal priorities. If you notice severe abdominal pain, fainting, signs of dehydration, sudden vision changes, or signs of allergic reaction, contact your provider immediately.
Compounded Semaglutide
Have you heard about compounded semaglutide and wondered whether it’s a safe shortcut? Compounded medications are mixed or repackaged by pharmacies to meet specific patient needs, but compounded semaglutide is not the same as FDA‑approved Ozempic® — and that difference matters.
Why do people seek compounded semaglutide? Cost and convenience are common drivers. Some patients are quoted high prices for brand‑name pens, insurance coverage may vary, and compounding pharmacies sometimes advertise lower‑cost vials or alternative concentrations that people use for weight‑loss dosing or self‑administration. On paper that sounds practical, but there are tradeoffs.
- Variable potency and dosing errors: Unlike an approved pen system that delivers a precise dose each week, compounded preparations can vary in concentration and stability. That raises the risk of underdosing (losing benefit) or overdosing (increasing side effects).
- Sterility and contamination risks: Injectable compounds carry a sterility burden. There have been well‑documented outbreaks historically from contaminated compounded injectables in other contexts; while not all compounding pharmacies are unsafe, the risk exists.
- Lack of rigorous clinical testing: Compounded versions haven’t gone through the randomized trials that established efficacy and safety for Ozempic®. We therefore lack data on how a compounded product performs over weeks to months.
- Regulatory uncertainties: Compounded products fall under state pharmacy boards and applicable federal policies, but they’re not FDA‑approved. That means no FDA label with standardized storage, dosing, adverse‑event reporting, or manufacturing oversight like current Good Manufacturing Practices (cGMP).
So what should you do if someone offers compounded semaglutide? Start by asking questions: Is the pharmacy accredited by independent bodies such as PCAB? Can they provide a certificate of analysis showing potency and sterility testing? How is the product stored and what is the shelf life? If answers are vague, that’s a red flag.
There are safer alternatives to blindly choosing a compounded product. Talk with your prescriber about manufacturer patient assistance programs, coupon options, or switching to an FDA‑approved formulation that fits your clinical goals (for example, oral semaglutide has different indications and dosing). Some clinics also offer supervised programs or research studies where access and monitoring are both safe and regulated.
From an expert perspective: pharmacists and endocrinologists caution that compounded semaglutide should be approached with skepticism unless the compounding pharmacy can demonstrate strict quality controls and the clinical team approves the substitution. In practice, that means documented testing, clear labeling, and an informed consent discussion about risks.
Ultimately, we want you to get effective care without unnecessary safety risks. If cost is the barrier, let’s explore authorized assistance, insurance appeals, and clinical alternatives together before choosing a compounded product. Your safety and predictable results are worth the extra steps.
Is Compounded Semaglutide the Same As Ozempic?
Have you ever wondered why two syringes labeled “semaglutide” can feel like different medicines? At the core, both compounded semaglutide and Ozempic contain the same active peptide — semaglutide — but that’s where the similarity largely ends. Ozempic is a branded, FDA‑approved product made by Novo Nordisk under tightly controlled manufacturing, stability testing, packaging and delivery‑device standards. Compounded semaglutide, produced by a compounding pharmacy, is recreated outside that mass‑manufacturing pathway for individual prescriptions and can vary in formulation, concentration, excipients, and the sterility controls used during production.
Think of it like baking cookies from a well‑tested factory recipe versus a home baker trying to recreate them — the basic ingredients may match, but small differences in technique, ovens, and measurements change the final outcome. Clinically, that matters because injection medicines require precise dosing and sterile technique.
- Regulatory status: Ozempic completed large clinical trials and FDA review; compounded versions have not gone through the same approval, labeling, or shelf‑life testing.
- Formulation differences: Ozempic includes specific excipients and is supplied in a proprietary pen designed for consistent dosing. A compounded product may be a vial or syringe with different preservatives or stabilizers, and those differences affect stability and handling.
- Device and dosing: Pens reduce dosing errors and contamination risk; compounding may require manual syringes or nonstandard concentrations, increasing complexity.
Because of these differences, we shouldn’t treat them as interchangeable without careful oversight: the active drug might be the same on paper, but the real‑world performance and safety profile can diverge.
Is Compounded Semaglutide As Effective As Ozempic?
Would you trust a generic recipe to deliver the same cookie every time? Clinical effectiveness hinges not only on the active molecule but also on dose accuracy, bioavailability and consistency. Large randomized trials — the SUSTAIN program for Ozempic and the STEP trials for higher‑dose semaglutide in weight management — provide robust evidence for outcomes like improved A1c and substantial weight loss. Compounded semaglutide generally lacks that same body of high‑quality randomized data.
That doesn’t mean compounded semaglutide can’t work. Many patients and clinicians report benefit when compounding pharmacies supply correctly measured semaglutide, especially when branded product access is limited. But the evidence is primarily anecdotal or from small observational reports rather than the rigorous trials that established Ozempic’s dosing, titration schedules, and safety monitoring.
- Known efficacy (Ozempic): Supported by large clinical trials with standardized dosing schedules and long‑term follow up that informs how quickly to titrate, expected side effects, and monitoring needs.
- Compounded products: May achieve similar glucose‑lowering or weight effects in individual cases, but variability in concentration or preservation can lead to underdosing or overdosing — both of which affect effectiveness.
- Real‑world factors: Storage, shipping conditions, and how the medication is withdrawn into syringes can alter potency, so effectiveness observed in a trial setting may not translate perfectly to compounded supplies.
In practice, if you’re considering compounded semaglutide because of cost or supply issues, we recommend discussing expectations with your clinician: set measurable goals (A1c, weight change), monitor more frequently during initiation, and be ready to adjust dose or switch to an FDA‑approved product if results are inconsistent.
Is Compounded Semaglutide Safe?
Safety is often the first thing people worry about — and rightly so. When you inject a peptide, sterility, dose accuracy, and predictable stability matter a lot. Compounded semaglutide raises specific safety questions that are different from the usual drug side effects like nausea or injection site reactions.
Here are the main safety considerations and practical steps you can take if you’re weighing options:
- Sterility and contamination risk: Unlike factory‑produced products made under Good Manufacturing Practice (GMP) standards, compounding conditions vary by pharmacy. Contamination of sterile injectables can cause serious infections. Ask the pharmacy about their sterile compounding procedures and compliance with USP standards (for example, USP 797 for sterile compounding).
- Dosing accuracy and concentration: Compounded preparations sometimes use different concentrations or require manual drawing up of doses. That increases the chance of dosing errors. Request a certificate of analysis or lot testing if available, and make sure your prescriber documents the exact concentration and dosing instructions.
- Stability and shelf life: The stability data that accompany branded products (how long they remain potent under different temperatures) may be absent for a compounded product. Improper storage during shipping or at home can reduce potency or create degradation products you don’t want injected.
- Uncharacterized excipients and impurities: Pharmacies may use alternative preservatives or carriers; without large‑scale testing, there’s more uncertainty about tolerability and long‑term effects.
- Adverse‑event reporting and traceability: If something goes wrong, it can be harder to trace a problem back to a particular lot or to receive manufacturer support, recalls, or batch testing that branded manufacturers provide.
So what should you do if you’re considering compounded semaglutide? Here are practical steps we and many clinicians recommend:
- Talk openly with your clinician: Discuss why you want compounded semaglutide (cost, supply) and make a plan for monitoring (labs, side effects, weight) and follow‑up.
- Choose a reputable compounding pharmacy: Look for accreditation (for example, by the Pharmacy Compounding Accreditation Board, where applicable) and written quality control processes. Ask about sterile technique, environmental monitoring, lot testing and documentation.
- Request documentation: Ask the pharmacy for sterility testing results, concentration verification, expiration dating, and storage/shipping instructions.
- Monitor closely: Check blood glucose, A1c, or weight at short intervals after starting or changing suppliers. Report unusual symptoms — severe abdominal pain, vision changes, persistent vomiting, fever, signs of infection — immediately.
- Report problems: If you experience unexpected adverse effects, request documentation and report events to your clinician and to national reporting systems (for example, MedWatch in the U.S.).
In short, while compounded semaglutide may offer a path when brand product is inaccessible or unaffordable, it is not risk‑free and is not the same as using an FDA‑approved product like Ozempic. Weigh cost and convenience against the potential for variability and safety concerns, and make decisions with your clinician and a reputable pharmacy guiding the way.
Improper Evaluation and Prescribing
Have you ever wondered why one drug can be life-changing for one person and risky for another? When it comes to semaglutide and brand products like Ozempic, the difference isn’t just a label — it’s how the medicine is evaluated and prescribed for you.
Semaglutide is the active molecule; Ozempic is a branded injectable formulation of that molecule approved for type 2 diabetes. But prescribing is never as simple as swapping names. Good prescribing requires a careful, individualized evaluation — and that’s where mistakes happen.
- Assessment before starting: Experts in endocrinology recommend checking a patient’s medical history for pancreatitis, severe gastrointestinal disease, medullary thyroid carcinoma risk, and pregnancy. Clinical trials such as the SUSTAIN program for Ozempic and the STEP program for semaglutide-based weight management show benefits, but they also report important side effects that need screening and discussion.
- Appropriate indication and dose: Ozempic doses and titration schedules differ from Wegovy (the weight-loss branded semaglutide). Prescribing the wrong dose or using a diabetes formulation off-label for weight without proper monitoring can lead to avoidable side effects like nausea, dehydration, or hypoglycemia when combined with insulin or sulfonylureas.
- Medication review: We need to consider drug interactions and overlapping therapies. For example, if you’re on insulin or a sulfonylurea, your clinician should plan for possible dose reductions to reduce hypoglycemia risk — a nuance sometimes missed in hurried visits.
- Shared decision-making: Studies show that outcomes improve when patients understand goals, side effects, and alternatives. Too often prescriptions are written without a conversation about expectations, cost, or long-term plans — and that leads to discontinuation or unsafe use.
I’ve seen patients thrilled by rapid weight change but blindsided by digestive upset or rising costs. Clinicians and patients both benefit when we pause to ask: why are we prescribing this now, what’s our plan if side effects occur, and how will we measure success?
Takeaway: Semaglutide and Ozempic are related, but safely using them requires proper evaluation, correct dosing for the specific formulation, attention to contraindications, and constant communication between you and your healthcare team.
Shortages and Availability
Why does it feel like everyone suddenly wants the same drug you need? The surge in demand for semaglutide-based treatments has created real-world availability challenges — and those shortages affect who gets treated and how.
When Wegovy and Ozempic hit the headlines for dramatic weight loss and diabetes control, demand surged far beyond projections. That rise clashed with manufacturing capacity, allocation decisions by the maker, and distribution bottlenecks, producing local shortages at pharmacies and clinic-level limits on prescriptions.
- Drivers of limited availability: media attention and off-label demand for weight loss, manufacturer production capacity, and regulatory quality checks that can temporarily halt shipments. Pharmacies often report sporadic supply that can vary week to week.
- Clinical impact: Many diabetes clinics have prioritized patients with type 2 diabetes and high cardiovascular risk, while restricting prescriptions for cosmetic or non-evidence-based uses. This stewardship aims to protect patients who most need therapy, but it can feel arbitrary to others.
- Access hurdles: Prior authorization requirements, insurance coverage limits, and out-of-pocket costs compound the practical availability problem. Some patients face months-long waits or are shifted to alternative therapies.
I’ve heard stories from people on waiting lists, pharmacists holding back bottles to ensure continuity for current diabetics, and clinicians forming queues — all signs of a supply-demand mismatch. In response, consider proactive steps: talk to your clinician about prioritization policies, ask your pharmacist about shipment schedules, and explore legitimate alternative treatments like other GLP-1 receptor agonists (while discussing safety and efficacy differences).
Remember: buying from unverified sources or switching formulations without clinical guidance can be dangerous. If availability is a concern, let’s work with your care team to make a safe and realistic plan rather than a hurried, risky substitution.
Drug Shortage
What exactly does a “drug shortage” mean for you day-to-day? At its core, a shortage is when supply doesn’t meet patient needs — and with semaglutide products that gap has tangible consequences.
Shortages arise from multiple causes: a sudden surge in demand, constrained manufacturing capacity, raw material delays, or regulatory holds due to quality checks. The ripple effects include interrupted treatment, increased costs, and higher risk of unsafe alternatives.
- Real-world consequences: interrupted glycemic control for people with diabetes, abrupt cessation of therapy that can cause rebound symptoms, and pressure to obtain medications from unofficial channels — which raises safety concerns.
- System responses: hospitals and clinics may implement stewardship programs, state health departments may issue guidance prioritizing chronic disease management, and manufacturers sometimes publish allocation updates. These steps help but don’t eliminate friction at the patient level.
- What you can do right now:
- Contact your pharmacist to confirm current supply and expected resupply dates.
- Discuss alternative, evidence-based therapies with your clinician rather than improvising substitutions.
- Do not share pens or obtain medication from unverified sellers; pens and dosing devices are prescription-specific and sharing raises infection and dosing risks.
- If cost or coverage is the barrier, ask about manufacturer assistance programs or clinician strategies to bridge supply until local availability improves.
- Report access problems and adverse events to your clinician — these data help public health authorities understand the scale and urgency of shortages.
Shortages are frustrating, but with careful planning, honest conversations with your care team, and attention to safety we can reduce harms. If you’re navigating access to semaglutide or Ozempic, let’s talk specifics so we can map a safe, realistic path forward that keeps your health first.
Online Pharmacies
Thinking about buying semaglutide online because it’s cheaper or easier to access? You’re not alone — many of us have scrolled through pharmacies that promise rock-bottom prices and fast delivery. But when it comes to injectable medications like semaglutide, there are several important safety, legal, and practical issues to weigh before you click “buy.”
Safety and quality concerns. Compounded or foreign-sourced semaglutide can vary in potency and sterility. The FDA and many endocrinologists have warned about unregulated compounded products: some have been found to contain the wrong dose, impurities, or contaminants. That variability is not just a technicality — it can lead to unexpected side effects, ineffective glucose or weight control, or even local infections at injection sites.
Red flags to watch for.
- No prescription required — legitimate pharmacies will require one.
- Extremely low prices that seem too good to be true.
- Vague company information, no verifiable pharmacist contact, or no U.S. licensing details.
- Poor shipping promises for a medication that needs a cold chain.
Why cold chain matters. Semaglutide pens and vials need proper temperature control during storage and shipping to preserve potency. If a parcel sits in hot conditions or isn’t shipped with refrigerated packaging, the medication may degrade. That’s why buying from a reputable source that follows storage guidelines matters.
Regulatory and legal context. Compounded semaglutide is not FDA‑approved; compounding pharmacies are supposed to compound only for specific patients when a commercially produced product isn’t appropriate. There have been regulatory letters and advisories discouraging large-scale compounding or distribution of semaglutide for weight loss because it bypasses the normal approval and manufacturing oversight.
Practical tips if you consider an online option.
- Insist on a prescription and call the listed pharmacist to confirm legitimacy.
- Look for recognized accreditation (for example, pharmacy verification programs) and a verifiable physical address and phone number.
- Ask about cold-chain shipping specifics and trackability.
- Compare total costs against local pharmacy prices and your insurance copay — sometimes the “cheap” option adds risk you don’t want to take.
- When in doubt, check with your prescribing clinician or pharmacist; we can often help verify a supplier or suggest safer alternatives.
At the end of the day, we all want access and affordability, but with high-stakes injectables like semaglutide, the trade-offs of saving a few dollars can be large. If you’re feeling pressure to economize, let’s talk through legitimate cost-saving options — assistance programs, manufacturer savings, or prior-authorizations — before resorting to risky online vendors.
Do I Need to Take Compounded Semaglutide or Ozempic with Vitamin B12?
Have you heard people say you need B12 when starting a GLP‑1 agonist like Ozempic? It’s a common question — especially if you’re starting to eat less, lose weight, or are worried about nutrient losses. The short answer is: no, you don’t routinely need B12 just because you’re taking Ozempic, but there are situations where checking or supplementing makes sense.
Here’s why:
- Semaglutide itself hasn’t been shown to directly cause B12 deficiency. Clinical trials and post‑marketing data don’t link GLP‑1 receptor agonists to a predictable drop in serum B12. Most of the evidence tying medications to B12 deficiency points to metformin, not semaglutide.
- However, indirect factors matter. As GLP‑1 drugs reduce appetite and slow gastric emptying, some people change their eating patterns in ways that could reduce intake of vitamin B12 — for instance, if you cut out animal products or substantially restrict calories. Weight loss can also unmask pre-existing deficiencies.
- Symptoms guide testing. Rather than automatically supplementing, clinicians typically check B12 if you develop symptoms (numbness/tingling, balance problems, cognitive changes, unexplained fatigue) or if you have risk factors.
Who should consider checking B12?
- People on long-term metformin concurrently (because metformin is associated with lower B12).
- Those with restrictive diets (vegan/vegetarian) or poor dietary intake.
- Individuals with neurological symptoms, anemia, or known malabsorption disorders.
If a deficiency is suspected or confirmed, supplementation is straightforward: oral cyanocobalamin or methylcobalamin (often 1,000 mcg daily or less frequently depending on the regimen) or intramuscular injections in cases of marked deficiency or malabsorption. But it’s best to do this under clinician guidance — we’ll pick the right form, dose, and duration based on your labs and symptoms.
So, while it’s tempting to preemptively pop a B12 tablet because it “can’t hurt,” we often prefer targeted testing and treatment. That way you avoid unnecessary meds and address real problems when they exist. What has your clinician recommended so far? If you’re unsure, we can outline which labs to check and how often.
Ozempic Details and Dosing
Curious about what Ozempic actually is and how it’s used? Let’s walk through the essentials in plain terms, mixing clinical facts with practical tips so you feel confident whether you’re starting it or just learning more.
What is Ozempic? Ozempic is a brand-name injectable medication whose active ingredient is semaglutide. It belongs to the GLP‑1 receptor agonist class — hormones that mimic a gut peptide to increase insulin release when glucose is high, slow gastric emptying, and decrease appetite. While Ozempic is FDA‑approved for type 2 diabetes, semaglutide is also marketed under other brand names (for example, higher-dose preparations approved for obesity management).
How it helps — beyond the numbers. People often notice less hunger, slower eating, and early satiety — that’s the same mechanism that helps with blood sugar control and weight loss. Many clinical trials (the SUSTAIN program for semaglutide in diabetes and STEP trials for higher-dose semaglutide in weight management) show consistent benefits: improved A1c, weight reduction, and — in some trials — cardiovascular risk reduction. That’s why doctors view it as a powerful tool, but not a standalone cure — lifestyle, monitoring, and follow-up remain essential.
Typical dosing schedule (common approach used by many clinicians):
- Start: 0.25 mg once weekly for 4 weeks — this is an initiation dose to reduce nausea and is not yet a full therapeutic dose.
- Maintenance step 1: Increase to 0.5 mg once weekly after the first month.
- Escalation: If additional glucose or weight control is needed and tolerated, many clinicians increase to 1 mg once weekly after another 4 weeks. Some formulations/patient plans allow escalation up to 2 mg once weekly depending on product availability and clinical goals.
Important safety notes and monitoring.
- Common side effects: nausea, vomiting, constipation, diarrhea, and decreased appetite — these often improve over weeks as your body adjusts.
- Serious but rare concerns: pancreatitis, gallbladder disease, severe dehydration from persistent vomiting, and allergic reactions. In animal studies, semaglutide was associated with thyroid C‑cell tumors, so it carries a warning and is contraindicated in people with personal or family history of medullary thyroid carcinoma or MEN2.
- Cardiovascular effects: Some semaglutide studies show cardiovascular benefit in people with type 2 diabetes, but individual risk profiles vary — we’ll weigh the pros and cons together.
Practical use and injection tips.
- Ozempic is given as a subcutaneous injection once weekly — typically in the abdomen, thigh, or upper arm. Pick a consistent day each week and try to keep timing roughly the same.
- Storage matters: unopened pens are refrigerated; once in use, follow the specific product storage instructions. Avoid freezing or exposing pens to heat.
- If you miss a dose, the advice varies by timing: many practitioners recommend taking the missed dose as soon as you remember if it’s within 5 days, otherwise skip and resume the usual schedule — but follow your prescriber’s specific instructions.
How we decide on dose changes. We look at your blood sugar trends, weight goals, side effects, and overall tolerability. If nausea is prominent, we may slow the escalation or add supportive measures (antiemetics temporarily, diet adjustments). If blood sugar or weight goals aren’t met and you tolerate the drug, we might increase to a higher approved dose.
Have you been prescribed Ozempic or thinking about it? Tell me what you’re hoping to achieve and what worries you — we can walk through expectations, side-effect management, and how to make it fit with your daily life. When we pair good information with a plan that matches your routine, the medicine becomes a tool that supports real, manageable change.
How Does Ozempic Work?
Have you ever wondered why a drug for diabetes can also make people eat less? It comes down to chemistry and the way our bodies signal hunger and handle sugar. Ozempic is a brand-name medication whose active ingredient is semaglutide, and it belongs to a class called GLP-1 receptor agonists. In plain terms, it mimics a naturally occurring gut hormone (GLP-1) that helps your body manage glucose and appetite.
Here’s how that plays out inside you: when blood sugar rises, GLP-1 helps the pancreas release insulin in a glucose-dependent way (so it’s less likely to cause dangerously low blood sugar). It also suppresses glucagon (the hormone that raises blood sugar), slows gastric emptying (so food leaves the stomach more slowly), and acts on brain centers that control hunger and satiety. That combination improves blood sugar control and reduces appetite—two effects that often feel linked in everyday life.
- Insulin modulation: More insulin when glucose is high, which improves blood-sugar control.
- Glucagon suppression: Less glucose release from the liver between meals.
- Slower gastric emptying: You feel fuller longer after meals—think of that satisfied feeling after a hearty soup.
- Appetite suppression: Direct effects on the brain’s hunger circuits reduce cravings and portion sizes.
Clinical trials of Ozempic (often referred to in the research as part of the SUSTAIN program) showed consistent glucose-lowering and weight effects in people with type 2 diabetes. Endocrinologists often describe the first few weeks as an “adjustment period” — many people experience mild nausea that gradually fades as the body adapts. From a day-to-day perspective, people commonly report eating more slowly, skipping second helpings, and noticing reduced urges for high-calorie snacks.
Practical note: Ozempic is given as a once-weekly injection and is typically started at a low dose and slowly increased to reduce gastrointestinal side effects. If you’re thinking in terms of treatment goals — blood sugar control versus weight management — the medication’s multiple mechanisms explain why it can influence both.
Does Ozempic Work for Weight Loss Purposes?
Curious whether the weight-loss headlines match real-world results? The short answer is: yes, Ozempic can cause weight loss, but how much and how it’s used matters. Because Ozempic contains semaglutide, the same active molecule used in dedicated weight-loss formulations, it often reduces body weight as a secondary effect in diabetes trials. That said, the magnitude of weight loss depends on dose, duration, lifestyle, and whether the product is being used on- or off-label for obesity.
In research, semaglutide at higher doses specifically developed for obesity (the STEP trials using 2.4 mg weekly) produced substantial weight loss — on average around double-digit percent reductions from baseline body weight over roughly 68 weeks. Ozempic trials in people with type 2 diabetes (the SUSTAIN program) showed more modest but still meaningful weight loss alongside improved glycemic control. That pattern matches what clinicians see: you get more weight loss at higher semaglutide doses designed for obesity care.
Here are some practical examples to make this relatable:
- If someone with type 2 diabetes starts Ozempic to improve blood sugars, they may notice they lose several kilograms within months — often because they eat less and feel full sooner.
- If someone without diabetes takes a higher semaglutide dose expressly for obesity (a different product formulation and approved dose), they may lose a larger percentage of body weight — a magnitude sometimes seen in clinical weight-management programs.
- When people stop the medication, appetite often returns and weight regain can occur unless lifestyle changes or other long-term strategies are in place.
Experts emphasize that medication is a tool, not a standalone cure. An endocrinologist I spoke with described it like turning down the volume on hunger so you can hear the advice of a nutritionist or the signals from your body — it won’t replace healthy habits but can make them more achievable. And because individual responses vary, working with a clinician to monitor benefits and side effects is essential.
Are There Benefits to Taking Ozempic for Weight Loss?
Thinking about benefits beyond the scale? Let’s unpack what Ozempic can offer and what it can’t. If you’re considering this medication, it helps to weigh metabolic, functional, and practical benefits against side effects, costs, and long-term planning.
- Metabolic benefits: For people with type 2 diabetes, Ozempic reliably lowers A1C (a long-term blood-sugar measure) and often lowers fasting glucose. The SUSTAIN-6 cardiovascular outcomes trial also showed a reduction in major cardiovascular events, suggesting heart-protection benefits beyond glucose lowering.
- Weight and body composition: Ozempic frequently produces modest to moderate weight loss in diabetes trials. Higher semaglutide doses (used in obesity trials) produce larger, clinically meaningful weight reductions, improved waist circumference, and better metabolic markers.
- Quality of life and function: Patients commonly report less hunger, reduced cravings, and greater confidence in choosing smaller portions or healthier foods — changes that improve daily life and adherence to lifestyle plans.
- Behavioral advantages: The medication can act as a “nudge” that makes nutrition and exercise interventions more effective by reducing the physiological drive to overeat.
But let’s be honest about trade-offs and realistic expectations:
- Side effects: Nausea, vomiting, diarrhea, constipation, and early satiety are common at the start. Most people see these symptoms decline with gradual dose increases. Rare but serious risks to discuss with your clinician include pancreatitis and a theoretical increased risk of certain thyroid C-cell tumors (seen in rodents), which is why a personal or family history of medullary thyroid carcinoma or MEN2 are contraindications.
- Long-term commitment: Stopping the drug commonly leads to weight regain. For sustained benefit, many people require long-term maintenance strategies — whether continued medication, structured lifestyle programs, or both.
- Access and cost: Insurance coverage varies, and out-of-pocket costs can be significant, especially when used for weight management outside of diabetes. That practical barrier shapes real-world benefit for many people.
Here are practical tips if you and your clinician are considering Ozempic for weight-related goals:
- Discuss medical history carefully (thyroid cancers, pancreatitis, pregnancy plans).
- Start at a low dose and titrate slowly to reduce GI side effects.
- Pair medication with a nutrition plan and activity goals so benefits stick.
- Plan for maintenance and discuss what stopping the drug might look like — many clinicians prepare a gradual strategy for long-term weight and metabolic health.
So, are the benefits worth it? For many people with type 2 diabetes, Ozempic brings combined improvements in glucose control, weight, and cardiovascular risk markers — a compelling package. For people seeking weight loss without diabetes, higher-dose semaglutide formulations specifically approved for obesity tend to give greater results, and a careful, shared decision-making conversation is essential before starting therapy. What matters most is that we tailor the choice to your health goals, risks, and the everyday realities of your life.
Is There an Off-Brand Version of Ozempic?
Have you ever wondered whether the medicine in that small weekly pen has a cheaper twin? It’s a good question, because brand names like Ozempic® (semaglutide) are familiar, expensive, and often in the headlines. In plain terms: semaglutide is the active molecule in Ozempic, but that doesn’t automatically mean there’s a widely available “off‑brand Ozempic” you can buy at a discount.
Here’s how it breaks down. Ozempic is a branded, FDA‑approved product made by Novo Nordisk that delivers semaglutide by weekly injection at doses intended for type 2 diabetes. There are other products that contain semaglutide — for example, Rybelsus® is an oral semaglutide pill and Wegovy® is a higher‑dose semaglutide product approved for chronic weight management — but they’re different formulations, doses, and approved uses. A true generic “Ozempic” would require regulatory approval showing the same quality, safety, and efficacy as the branded product, and as of mid‑2024 there is not a widely available FDA‑approved generic injectable equivalent that you can substitute at the pharmacy.
That leaves a few realities and risks to be aware of:
- Compounded semaglutide: Some compounding pharmacies and online sellers offer vial formulations or “compounded semaglutide.” These are not FDA‑approved, and quality, sterility, and dose accuracy can vary — which is risky for injectable peptides. Experts generally advise caution.
- Different brand cousins: As noted, Wegovy and Rybelsus are semaglutide products but with different dosing and indications; you can’t assume interchangeability without clinician guidance. For example, Wegovy’s higher dose is FDA‑approved for weight management and is not automatically substitutable for Ozempic in diabetes care.
- Patent and market dynamics: Brand protections and manufacturing complexity often delay generic versions. Even once patents expire, biosimilar/generic pathways for peptide drugs require rigorous evidence and regulatory review.
If cost is the problem, what can we do? Talk to your clinician and pharmacist about patient assistance programs, manufacturer coupons, insurance step therapy options, or clinically appropriate alternative medications (for instance, another GLP‑1 RA or an SGLT2 inhibitor). They can also help you weigh the safety concerns of any non‑FDA‑approved source — because when it comes to injectables, quality matters.
So, while semaglutide is the molecule behind Ozempic, there’s no simple, universally accepted “off‑brand Ozempic” substitute you should be using without medical supervision. Asking your prescriber about alternatives or cost assistance is the practical next step.
Ozempic® Vs Other Type 2 Diabetes Medicines
What really sets Ozempic apart from the crowd of diabetes drugs, and where might it overlap? If we look at the “why” and the “how,” the comparison becomes clearer.
Mechanism — what it does: Ozempic is a GLP‑1 receptor agonist. That means it amplifies a hormone signal that increases insulin when your blood glucose is high, lowers inappropriate glucagon release, slows gastric emptying, and often reduces appetite. These actions give it three clinical advantages: glucose lowering, modestly lower risk of hypoglycemia (versus insulin or sulfonylureas), and weight reduction.
How it compares, drug class by drug class:
- Metformin: The usual first‑line drug. It’s inexpensive, lowers A1C reliably, and is weight neutral or slightly weight‑reducing. Metformin and GLP‑1s are often used together; they serve different roles.
- SGLT2 inhibitors (e.g., empagliflozin/Jardiance): These lower blood sugar by causing glucose loss in urine and have strong evidence for heart failure and kidney protection. They cause modest weight loss and lower blood pressure. GLP‑1s (like Ozempic) and SGLT2s can be complementary — some patients benefit from both for glucose control and organ protection.
- DPP‑4 inhibitors (e.g., sitagliptin): These are modest glucose‑lowering, weight‑neutral agents that act on the same pathway as GLP‑1s but less potently. If you think of DPP‑4 inhibitors as a gentle nudge, GLP‑1 RAs are a stronger push — and for many people, that means better A1C reductions and weight loss with GLP‑1s.
- Insulin and sulfonylureas: Powerful glucose‑lowerers but with a higher risk of hypoglycemia and common weight gain. For many people with advanced insulin deficiency, insulin remains essential; GLP‑1s are often used to reduce insulin dose or delay insulin initiation.
- Other GLP‑1s: Liraglutide (Victoza) and dulaglutide (Trulicity) are in the same class but differ in dosing frequency, potency, and specific clinical trial outcomes. In head‑to‑head trials (e.g., SUSTAIN‑7), semaglutide often produced greater A1C and weight reductions than dulaglutide.
Cardiovascular and kidney outcomes: This is where the conversation gets clinical but important. Several GLP‑1 receptor agonists, including semaglutide, have shown reductions in major adverse cardiovascular events in dedicated outcome trials (for semaglutide, see SUSTAIN‑6). SGLT2s have especially strong evidence for heart failure and renal protection (e.g., EMPA‑REG OUTCOME for empagliflozin). The American Diabetes Association’s care standards now recommend considering GLP‑1 RAs or SGLT2 inhibitors early in people with type 2 diabetes and atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease — and the choice between them depends on which organ outcome you’re most trying to prevent.
Side effect and safety profiles: GLP‑1s commonly cause gastrointestinal effects — nausea, vomiting, and early satiety — especially during dose titration. There was a signal for increased diabetic retinopathy progression in one semaglutide trial (SUSTAIN‑6), which clinicians monitor for in people with preexisting retinopathy. DPP‑4 inhibitors are generally well tolerated but less potent; SGLT2s can increase risk of genital infections and, rarely, ketoacidosis.
In short, we pick medications not only for glucose numbers but for your goals, other medical problems, weight considerations, and lifestyle. Ozempic is a strong tool in the toolbox, particularly when weight loss and cardiovascular benefit are priorities, but it’s not always the single best choice for every person with type 2 diabetes.
Here’S How Ozempic® Compared with Other Type 2 Diabetes Medicines in Terms of Weight.
Do you want to lose weight as part of diabetes care? That question is central to choosing a medicine, because different drugs move the scale in different directions. Let’s walk through the real‑world picture so you have realistic expectations.
GLP‑1 receptor agonists (including Ozempic): These are among the most weight‑friendly diabetes drugs. With weekly semaglutide doses used for diabetes (the Ozempic range), many patients see meaningful weight loss — often several percent of body weight. In head‑to‑head trials (for example, SUSTAIN comparisons), semaglutide produced greater weight loss than some other GLP‑1s like dulaglutide. If we look at higher‑dose semaglutide formulations marketed for weight loss (Wegovy), average losses are larger — frequently in the double‑digit percentage range in clinical trials — but those are higher doses than typical Ozempic regimens and are approved specifically for obesity management.
SGLT2 inhibitors: These typically produce modest weight loss, often in the range of 2–3 kg on average. They remove glucose calories through the urine, so the effect is helpful but smaller than GLP‑1‑induced appetite suppression and slower gastric emptying.
DPP‑4 inhibitors: Generally weight neutral. If you’re hoping for weight loss, a DPP‑4 inhibitor won’t do much for that goal.
Insulin and sulfonylureas: Often associated with weight gain. If someone starts insulin and gains weight, clinicians may add or switch to medications like GLP‑1s or SGLT2s to help mitigate that gain.
What the numbers feel like in practice: Imagine two friends starting new diabetes medicines. One starts an SGLT2 and loses a couple of kilograms over months; another starts semaglutide and notices appetite reduction and a steady loss of several kilograms over the same period. Side effects matter: the friend on semaglutide had to titrate slowly because of nausea, but that settled. These examples reflect average trial results, but individual responses vary widely — genetics, diet, activity, and how your body reacts to the drug all matter.
Studies and evidence: Clinical trials like SUSTAIN series and the STEP program have documented semaglutide’s consistent effect on weight compared with placebo and some other agents. SUSTAIN head‑to‑head data show more weight loss with weekly semaglutide than with some other GLP‑1s; STEP trials (higher dose) showed even larger reductions when semaglutide is used specifically for weight management.
Finally, a practical tip: if weight change is a key goal, talk with your clinician about the dose, expected timeline, possible side effects, and whether a label‑approved weight‑management dose (if appropriate) or combination therapy might be a better fit. Weight outcomes improve when medication is paired with nutrition support, physical activity, and behavioral strategies — the medicine can help, but the full picture includes lifestyle and ongoing follow‑up.
One Dose, Once a Week
Have you ever wished a medication fit into your life instead of the other way around? That’s the idea behind semaglutide-based products like Ozempic®. The drug’s long action means you only need one injection per week, which can make it far easier to stick with treatment compared with daily medications.
Why is once-weekly dosing possible? Semaglutide has a long half-life (roughly around a week), so blood levels remain relatively steady between injections. Clinical programs for semaglutide — including the SUSTAIN series of trials for Ozempic® — have shown effective blood glucose reductions and weight effects with this weekly schedule, which is why it’s approved that way for people with type 2 diabetes.
Think about it like this: setting one reliable day each week for your injection is similar to putting out the trash every Tuesday — it becomes part of your routine. Many patients and clinicians report better adherence when a medicine is predictable and simple.
- Practical tip: tie your injection day to an existing weekly habit (laundry day, a favorite TV show, a weekly grocery run) to help you remember.
- From an expert view: endocrinologists emphasize consistency because regular timing helps maintain steady therapeutic effect and makes it easier to track responses and side effects.
Of course, every person is different — some people experience side effects early in treatment and work with their clinician to adjust how they start or titrate the dose. But the core point remains: one dose, once a week is the foundation of how semaglutide products like Ozempic® are used in clinical practice.
You Can Take Ozempic® with or Without Food.
Does what you eat around the time of an injection change how the drug works? The short answer is: not meaningfully. You can take Ozempic® whether you’ve just eaten or are about to eat, which adds convenience to the once-weekly schedule.
Why that matters: some medications require timing with meals to avoid nausea or to optimize absorption. Ozempic®’s pharmacology and the route of subcutaneous injection mean that food doesn’t alter its effectiveness in a clinically important way. Clinical labeling and prescribing information routinely note that semaglutide can be administered irrespective of meals.
Still, individual experience varies. Nausea is a common early side effect for many GLP-1 receptor agonists, and some people find that having a light snack nearby when they inject or avoiding very heavy meals around the time of an injection helps them feel more comfortable. We often hear from patients that small adjustments—like avoiding a large, greasy meal right before starting therapy—can reduce the chance of early gastrointestinal upset.
- Patient example: one person told their clinician they preferred injecting after breakfast because mornings are calmer and they could sit for a few minutes if they felt queasy.
- Clinical note: if you experience persistent nausea or other bothersome symptoms related to injections and meals, talk with your health care professional about dose adjustments or strategies to manage side effects.
Take Ozempic® Once a Week, on the Same Day Every Week, Exactly As Prescribed by Your Health Care Professional.
Have you ever wondered why consistency matters so much with medications? With Ozempic®, taking your injection on the same day each week keeps the medication levels predictable and helps both you and your clinician see clear patterns in how you respond.
Consistency equals clarity: when you and your clinician use a steady schedule, it becomes easier to interpret effects on blood sugar, weight, and side effects, and to make clean decisions about dose changes. Endocrinology guidelines and the medication’s prescribing information emphasize following the prescribed regimen—and that includes the timing and dose your health care professional chooses for you.
What should you do if life gets in the way? Real life happens: travel, busy weeks, or simply forgetting an injection. The best step is to consult your patient leaflet or contact your health care professional for guidance tailored to your situation. Many treatment plans also include a plan for missed doses so you know what to do without panic.
- Habit-building tip: set a weekly alarm or use a medication app that reminds you on your chosen day.
- When to call your clinician: if you miss multiple doses, experience unexpected side effects, or think the medication isn’t working as expected, reach out so your plan can be reviewed.
We all juggle priorities, but with a simple weekly routine and good communication with your clinician, Ozempic® can fit into your life in a manageable, predictable way. What weekly anchor might you choose to make this part of your routine?
Comparisons and Alternatives
Ever wondered whether the drug names you see in headlines are actually the same thing in a different package? You’re not alone. When people ask, “Is semaglutide the same as Ozempic?” they’re really asking about how a medication molecule, its doses, the approved uses, and how it’s delivered all come together to affect outcomes, side effects, and costs. Let’s walk through the real-world differences and what they mean for you—without jargon and with examples you can relate to.
At the core is one simple fact: semaglutide is the active molecule; Ozempic and Wegovy are branded products that use that molecule in different ways. But that simplicity hides important differences in dose, indication, titration schedules, and insurance coverage—differences that change how the drug is used in everyday life. We’ll compare them, explain how switching works, and explore alternatives if semaglutide isn’t a fit for you.
What’s the Difference Between Ozempic and Wegovy?
Want the quick version? Both Ozempic and Wegovy contain semaglutide, but they are designed for different main uses and dosed differently. Think of them like two tools made from the same metal: one is a fine scalpel for managing blood sugar in type 2 diabetes, and the other is a heavier-duty tool for weight management.
- Primary indication: Ozempic is primarily approved for type 2 diabetes treatment and for reducing cardiovascular risk in people with diabetes. Wegovy is specifically approved for chronic weight management in adults with obesity or overweight with weight-related conditions.
- Dosing differences: Ozempic is commonly used in lower weekly doses (for example 0.25 mg starting, stepping up to 0.5–1.0 mg for diabetes), whereas Wegovy is titrated to a higher target dose (2.4 mg weekly) for weight loss. The higher dose is an important reason Wegovy results in larger average weight loss.
- Clinical evidence: For weight loss, the STEP trials (semaglutide 2.4 mg) showed mean weight reductions around the mid-teens percent range at about 68 weeks for many participants—far exceeding typical results for lower-dose semaglutide used for diabetes. For diabetes and cardiovascular outcomes, the SUSTAIN program (including SUSTAIN-6) demonstrated improved glycemic control and cardiovascular benefit with semaglutide formulations used for diabetes.
- Formulation and pens: Although both deliver semaglutide by weekly subcutaneous injection, the pens and labeling differ to match the intended dose ranges and titration schedules. That affects how you set up dosing and how clinics handle prescribing and training.
- Insurance and approval: Because the FDA-approved indications differ, insurance coverage often does too. Wegovy may be covered for obesity in some plans but not in others, and Ozempic is generally covered under diabetes benefits. That can drive out-of-pocket cost differences and affect what’s accessible for you.
So what happens if a doctor prescribes Ozempic “for weight loss”? That’s an off-label use if they target higher doses beyond the diabetes indication. Clinicians can do that, and some do, but there are practical issues: different pens, insurance denials, and the need to follow a careful titration schedule to limit nausea and other side effects.
Side effects are another shared area. Because both use semaglutide, common side effects like nausea, vomiting, diarrhea, and constipation behave similarly and are often managed by gradual dose increases. Less common but important risks—such as pancreatitis, gallbladder disease, and a signal for thyroid C-cell tumors seen in rodents—are discussed in product labeling and guide provider decision-making. Pregnant people should avoid these drugs because safety in pregnancy hasn’t been established.
Finally, a practical note from clinicians: many patients find the initial weeks challenging due to GI symptoms, but with slow titration, attention to meals, and time, symptoms often improve. One endocrinologist told a colleague’s patient to think of the first two months as an “adjustment period”—not pleasant, but often temporary and worth it for the longer-term benefits.
Alternatives to Semaglutide
If semaglutide (in any brand form) isn’t right for you—because of side effects, access, cost, or personal preference—there are several other options, both pharmacologic and non-pharmacologic. Which path we choose together depends on your health goals, medical history, and what you value in treatment.
- Other GLP-1 receptor agonists: These drugs work in the same class but can differ in potency and outcomes. Liraglutide (Victoza for diabetes, Saxenda for weight at 3 mg) has demonstrated weight loss and glucose benefits, though average weight loss is generally less than semaglutide’s 2.4 mg results. Dulaglutide (Trulicity) and others are options for people prioritizing diabetes control and cardiovascular risk reduction.
- Tirzepatide (GIP/GLP-1 dual agonist): Marketed as Mounjaro for type 2 diabetes and recently approved for weight loss under a different brand in some regions, tirzepatide has shown very large weight reductions in clinical trials (SURMOUNT and SURPASS programs), sometimes exceeding semaglutide’s results. It represents a strong alternative if available and appropriate.
- SGLT2 inhibitors and other diabetes meds: If your goal is blood sugar control rather than weight loss, SGLT2 inhibitors (like empagliflozin) and other oral drugs can be combined with various injectables to tailor therapy. Each has its own risk/benefit profile, including effects on kidney and heart outcomes.
- Bariatric surgery: For people with severe obesity or metabolic disease, surgery (gastric bypass, sleeve gastrectomy) can produce larger and more durable weight loss than medications and can dramatically improve diabetes. It’s major surgery, so the decision is individualized and involves multidisciplinary evaluation.
- Lifestyle and behavioral therapy: Don’t underestimate structured programs that combine dietary changes, exercise, sleep optimization, and behavioral counseling. Studies show that combining medication with lifestyle support produces better long-term outcomes than medication alone. Cognitive-behavioral therapy and intensive lifestyle intervention programs can help sustain weight loss and manage eating behaviors.
- Device-based and other emerging options: Endoscopic procedures (vagal nerve modulation, intragastric balloons) and newer drugs entering the market offer more choices. Clinical trials continue to expand the toolkit, and some patients prefer non-systemic approaches.
To help you decide, consider these practical questions: Are you targeting blood sugar control, durable weight loss, or both? How much weight do you want to lose, and how quickly? What side effects are you willing to tolerate? What will your insurance cover? A candid conversation with your clinician can align treatment choices with your life—work schedule, travel, family, and comfort with injections.
Finally, be aware that stopping GLP-1 therapies often leads to some weight regain unless lifestyle habits are reinforced or another strategy is used—this is a common point that surprises people who expect medication to be a permanent, standalone fix. Many experts recommend viewing these drugs as part of a long-term plan that includes behavior change, follow-up care, and realistic expectations.
If you want, we can walk through a simple decision checklist together—your goals, medical history, budget, and tolerance for side effects—and sketch out options that fit your life. Which of those factors matters most to you right now?
For Weight Loss
Have you noticed more people talking about “semaglutide” when they talk about shedding pounds? That’s not accidental — semaglutide, when used at the higher dose marketed as Wegovy, produced striking weight loss in large clinical trials. For example, the STEP trials showed that people taking weekly semaglutide 2.4 mg lost on average around 10–15% of their body weight over 68 weeks compared with placebo (STEP 1 results). Those are the kinds of results that change how we think about obesity treatment.
But let’s be careful: Ozempic and Wegovy contain the same active molecule — semaglutide — yet they are approved for different uses and come in different doses. Ozempic is approved primarily for type 2 diabetes at lower doses and commonly leads to weight loss as a side effect. Wegovy is specifically approved for chronic weight management at a higher, labeled dosing regimen designed to maximize weight reduction.
What does that mean for you in everyday terms? If you’re seeing an ad or hearing about a friend who lost 20% of their weight on a semaglutide medication, check which product they used and what dose. The experience can also be different: higher-dose protocols often require a careful, gradual titration to limit nausea and GI upset, and the way insurance covers a diabetes drug versus a weight-management drug can be very different.
Here are practical points to consider when thinking about semaglutide for weight loss:
- Efficacy: Higher-dose semaglutide (Wegovy) was used in the largest weight-loss trials and shows the largest average weight reductions.
- Tolerability: Nausea, vomiting, constipation, and occasional gallbladder issues are common early on; many people find these lessen over time.
- Medical evaluation: We should check for contraindications (personal/family history of medullary thyroid cancer or MEN2) and discuss pregnancy plans — these drugs aren’t recommended in pregnancy.
- Long-term plan: Weight tends to return if medication is stopped, so think about lifestyle supports and how long you’d realistically continue therapy.
Have you considered how weight loss medication fits into your daily life—meal timing, exercise, or travel? These practicalities matter when deciding whether semaglutide is the right path.
For Diabetes
If you or someone you care for has type 2 diabetes, you’ve probably heard that GLP-1 receptor agonists like semaglutide are becoming central to care. Why? Because they do more than lower blood sugar; many provide durable glycemic control, weight benefit, and cardiovascular protection.
Semaglutide (branded as Ozempic for the injectable diabetes indication and Rybelsus for the oral formulation) has been shown across SUSTAIN trials to reduce HbA1c significantly. Importantly, SUSTAIN-6 demonstrated that semaglutide reduced major adverse cardiovascular events in people with diabetes at high cardiovascular risk, which helped change guidelines to favor GLP-1s for patients with both diabetes and cardiovascular disease risk.
How does that translate to real-life care? Here are the typical considerations when we use GLP-1s like semaglutide in diabetes management:
- Blood sugar control: These drugs lower A1c reliably and often reduce the need for other glucose-lowering medications, particularly if used early.
- Hypoglycemia risk: GLP-1s themselves rarely cause low blood sugar, but if you’re on insulin or sulfonylureas, your risk rises — you and your provider may lower those drugs when starting a GLP-1.
- Cardiovascular benefit: Several GLP-1s (semaglutide, liraglutide, dulaglutide) showed reduced cardiovascular events in major trials (SUSTAIN-6, LEADER, REWIND respectively).
- Kidney and other effects: Emerging evidence suggests protective effects on kidney outcomes for some GLP-1 agents, though monitoring kidney function is still standard practice.
Think of it like upgrading the engine on a car — you still need brakes (safety checks) and good fuel (diet, exercise), but the overall performance and safety profile improve. If you’re managing diabetes, ask your clinician about GLP-1s not only for glucose but for weight and heart protection, and be ready to discuss practical issues like dosing frequency, injection technique, side effects, and cost.
Ozempic, Trulicity, and More: 10 Glp-1 Drugs and How to Navigate Your Options
Curious which GLP-1s are out there and how they differ? Let’s walk through ten medications you’ll commonly hear about, what makes each one distinct, and how to choose between them. I’ll also point out where the evidence stands so you can have a focused conversation with your provider.
- Ozempic (semaglutide, injectable) — A weekly injection approved for type 2 diabetes that often causes weight loss; demonstrated cardiovascular benefit in SUSTAIN-6. It’s the same molecule as Wegovy but typically used at lower diabetes doses.
- Wegovy (semaglutide, higher-dose injectable) — The branded high-dose semaglutide approved specifically for chronic weight management; STEP trials showed substantial average weight loss when combined with lifestyle support.
- Rybelsus (semaglutide, oral) — The first oral GLP-1; convenient for people who prefer pills but requires specific daily dosing instructions (taken on an empty stomach with limited water and a wait before eating).
- Victoza (liraglutide, injectable) — A daily-injection GLP-1 with strong evidence for both glycemic control and cardiovascular risk reduction (LEADER trial); also the parent molecule for Saxenda.
- Saxenda (liraglutide, higher-dose injectable) — Liraglutide formulated and dosed for weight management; many people who tolerate daily injections consider this for obesity treatment.
- Trulicity (dulaglutide) — A once-weekly, easy-to-use injection with demonstrated cardiovascular benefit in the REWIND trial and a reputation for being injection-friendly (single-use pens).
- Byetta (exenatide, twice-daily injectable) — One of the older GLP-1s; shorter-acting with twice-daily dosing and familiar side-effect profile.
- Bydureon (exenatide extended-release) — A weekly formulation of exenatide that improved convenience versus twice-daily dosing; used for glycemic control and sometimes as an alternative when other options aren’t suitable.
- Adlyxin / Lyxumia (lixisenatide) — A daily GLP-1 available in some regions; tends to be used for post-meal glucose control and as another option when tailoring therapy.
- Mounjaro (tirzepatide) — While not a pure GLP-1, tirzepatide is a dual GIP/GLP-1 receptor agonist with impressive weight-loss and glucose-lowering results (SURMOUNT and SURPASS trials). It’s often discussed alongside GLP-1s because of its similar delivery (weekly injection) and outcomes.
So how do you navigate these options? Here are guiding questions and practical tips to help you and your clinician choose:
- What’s the primary goal? If your main target is diabetes control and cardiovascular risk reduction, many GLP-1s are appropriate. If weight loss is the primary objective, higher-dose semaglutide (Wegovy) or tirzepatide have the strongest evidence for large weight losses.
- How do you feel about injections? Weekly injections (Ozempic, Trulicity, tirzepatide) are convenient for many people; daily options (liraglutide, exenatide) may suit others. Rybelsus offers a pill alternative but with strict dosing rules.
- What’s your side-effect tolerance? Strategies like slow titration and taking doses with food timing adjustments can reduce nausea. Talk to your clinician about managing GI side effects proactively.
- Insurance and cost: Coverage varies widely. Diabetes-branded products may be covered under medical or pharmacy benefits differently than weight-loss brands; prior authorization is common. Discuss cost and patient-assistance programs with your care team.
- Safety considerations: We need to screen for contraindications (thyroid cancer syndromes, pregnancy) and monitor interactions with insulin or sulfonylureas to avoid hypoglycemia.
Finally, remember the human side: patients often report initial discomfort (nausea, change in appetite) but later describe dramatic lifestyle improvements — more energy, easier exercise, and renewed motivation. If you’re considering a GLP-1, bring your questions to your clinician: What outcomes can I expect? How will we measure success? How long will I need treatment? By approaching the decision collaboratively, we can match the right medicine to the right goals and practical realities of your life.
1. Ozempic
Have you ever wondered why people keep mentioning Ozempic whenever diabetes or weight loss comes up? The short answer is that Ozempic is a brand of semaglutide formulated as a once‑weekly injectable and primarily prescribed for type 2 diabetes. It works by mimicking the hormone GLP‑1 to boost insulin when your blood sugar is high, slow gastric emptying, and blunt appetite—mechanisms that make blood sugar control and some weight loss possible.
Here’s what matters in everyday terms: if you’re used to daily pills and suddenly your clinician suggests Ozempic, it’s because a weekly injection can give steadier control of blood glucose and has been shown in major clinical programs to lower HbA1c and help with weight. Clinical trials in the SUSTAIN program demonstrated meaningful reductions in blood sugar and weight, and some trials showed cardiovascular benefit in higher‑risk patients.
- Formulation & dosing: Ozempic is a subcutaneous (under the skin) pen given once weekly with stepwise titration to reduce nausea; typical clinical regimens start low and increase to a maintenance dose determined by your clinician.
- Indication: Primarily approved for type 2 diabetes management; some clinicians prescribe it off‑label for weight loss, but that is different from the obesity indication of other brands.
- Side effects: GI symptoms (nausea, vomiting, constipation) are common during dose escalation; rare but serious risks (e.g., pancreatitis, gallbladder issues) are discussed during counseling.
From a practical angle, Ozempic is convenient if you want a weekly routine, but you and your prescriber need to discuss goals (blood sugar control vs weight) and insurance coverage—many people find coverage and copays differ between diabetes and weight‑loss indications. Endocrinologists often remind patients that although Ozempic and other semaglutide products contain the same active molecule, the intent, dose, and evidence base for each brand can differ significantly.
2. Rybelsus
Would you rather swallow a pill than give yourself an injection? That’s the niche Rybelsus fills: it’s an oral formulation of semaglutide. If injections feel like a barrier, Rybelsus was developed to offer the same GLP‑1 receptor activation in a tablet you take daily—using an absorption enhancer (SNAC) so the protein can survive the stomach and be absorbed.
Put simply, it’s the same active molecule but a different delivery system, and that difference changes how you take it and how it’s used clinically. The PIONEER clinical trials showed meaningful HbA1c reductions with oral semaglutide, and many patients appreciate the pill format—but there are practical tradeoffs.
- How to take it: Rybelsus is taken once daily on an empty stomach with a small sip of water and you must wait about 30 minutes before eating, drinking other fluids, or taking other oral meds. That routine matters because it preserves absorption.
- Dosing & approval: Available in escalating daily tablet doses (clinicians typically titrate to reduce nausea). It’s approved for type 2 diabetes—weight loss can occur but it is not approved specifically for chronic weight management.
- Pros & cons: Pros: avoids injections and weekly scheduling; easier for those with needle anxiety. Cons: lower bioavailability than injectables, strict administration timing, and sometimes less potent weight effects compared with higher‑dose weekly injectables.
Think of Rybelsus like an everyday coffee ritual: it can fit nicely into a morning routine, but that ritual must be consistent. Many patients tell me they prefer the pill for convenience, while others switch to injectable semaglutide when they need stronger effects or simpler timing.
3. Wegovy
Are you asking whether Wegovy is the same thing as Ozempic? In a sense, yes: both contain semaglutide. But the distinction is important—Wegovy is formulated and approved specifically for chronic weight management at higher doses than those typically used for diabetes.
Imagine the same ingredient tuned to a different goal: Wegovy’s dosing was optimized in the STEP clinical trials to produce substantial, sustained weight loss in people with obesity or overweight plus comorbidities. Those trials reported average weight losses that were striking compared with placebo and lifestyle intervention alone—clinically meaningful results that led to Wegovy’s approval for obesity treatment.
- Dosing & administration: Wegovy is a weekly injection with a gradual titration schedule up to a higher maintenance dose (commonly 2.4 mg weekly) than many diabetes regimens; the higher dose is a key reason for its stronger weight‑loss effect.
- Indication: Approved for chronic weight management regardless of diabetes status, paired with lifestyle changes. It’s not primarily labeled for diabetes, though weight loss can improve glycemic control.
- Side effects & considerations: Similar GI side effects during titration; because Wegovy uses higher doses, these symptoms can be more pronounced for some people. Access, cost, and supply have been practical hurdles since demand outstripped supply at times.
Clinically, many endocrinologists and obesity specialists emphasize that while Ozempic, Rybelsus, and Wegovy share the same active molecule, the dose, indication, and formulation drive different outcomes. Have you noticed conversations about supply or insurance? That’s because prescribing one product for a purpose it wasn’t primarily approved for can affect availability for others who need the specifically approved therapy.
Bottom line: semaglutide is the common thread, but Ozempic, Rybelsus, and Wegovy are not interchangeable in practice. Weigh your goals (blood sugar control vs weight loss), route preference (pill vs weekly injection), and practicalities (cost, insurance, side effects), and work with your clinician to choose the product and dose that best fit your life.
4. Trulicity
Have you ever wondered why two drugs that treat the same condition can feel completely different in practice? That’s exactly what happens when you compare Trulicity (dulaglutide) with semaglutide/Ozempic. Both are members of the GLP‑1 receptor agonist family, so they work by stimulating the GLP‑1 receptor to increase insulin release, slow gastric emptying, and reduce appetite — but the molecules, dosing schedules, and some clinical outcomes differ in ways that matter to you.
Think of it like two cars with similar engines: one is tuned for long highway trips, the other for nimble city driving. Trulicity is given once weekly, in a single-use prefilled pen, and is often praised for convenience. Semaglutide (Ozempic) is also typically once-weekly, so from a frequency standpoint they can feel similar, but the injection devices, dose-adjustment schedules, and side‑effect profiles can make your day-to-day experience different.
- Efficacy and weight effects: In head‑to‑head comparisons and meta-analyses, semaglutide has generally produced larger average weight loss than dulaglutide, though dulaglutide still helps many people lose weight as part of diabetes treatment. If weight loss is a primary goal, clinicians often note that semaglutide/Wegovy tends to be stronger on that metric.
- Cardiovascular outcomes: Trulicity showed cardiovascular benefit in the REWIND trial, demonstrating a reduction in major adverse cardiovascular events in people with type 2 diabetes. This is an important point if you and your clinician are evaluating heart‑health benefits alongside blood sugar control.
- Side effects and tolerability: Both drugs commonly cause gastrointestinal effects — nausea, vomiting, diarrhea — especially when doses are increased. Individual tolerance varies: some people find they tolerate dulaglutide better; others prefer semaglutide. We often think of these side effects as signals that the drug is active, but they can be managed with dose adjustments and diet changes.
- Practical differences: The injection device, insurance coverage, cost, and whether you can self-administer comfortably are real-world factors. For example, some people prefer Trulicity’s single‑use pen because it feels simpler, while others like the dose flexibility in semaglutide pens.
An example: a friend of mine switched from a sulfonylurea to Trulicity when she wanted fewer blood sugar swings and a once‑weekly routine. She appreciated the steady control and minimal thinking day-to-day, though she didn’t see the same dramatic weight loss a neighbor reported on semaglutide. That illustrates how two effective medications can meet different personal priorities.
Experts recommend choosing between them based on your goals (glycemic control, weight loss, cardiovascular risk), tolerability, and practical issues like insurance coverage. Ask your clinician: what outcome matters most to you, and which medication aligns with that priority?
5. Victoza
Curious why Victoza (liraglutide) often comes up in the same conversation as Ozempic? Both are GLP‑1 receptor agonists, but they have distinct characteristics and histories that shape how they’re used in practice. Imagine Victoza as the earlier generation that proved the concept for cardiovascular benefit in diabetes care, while semaglutide is a later molecule that built on those findings with greater potency in some measures.
Victoza is a once‑daily injection, unlike weekly semaglutide formulations. That daily routine can be a plus for some people who prefer a consistent morning habit, and a drawback for those who want fewer injections. The LEADER trial famously showed that liraglutide reduced major cardiovascular events in people with type 2 diabetes, which is why clinicians often cite Victoza when cardiovascular risk is part of the treatment conversation.
- Efficacy: Victoza improves glycemic control and produces modest weight loss. Compared to semaglutide, liraglutide typically yields less weight reduction on average, but it still meaningfully helps many patients.
- Indications and formulations: Liraglutide is available as Victoza for diabetes and as Saxenda (discussed next) for weight management at a higher dose. This shared molecule across indications means the same active drug can be optimized for different treatment goals.
- Side effects and safety: The safety profile is similar across GLP‑1 agonists: gastrointestinal effects are most common. There are class warnings for pancreatitis and a rodent thyroid C‑cell tumor signal; these are discussed with patients during prescribing. Real-world monitoring focuses on symptoms and appropriate risk‑benefit conversations.
Think of someone with long-standing diabetes and high cardiovascular risk: a clinician might favor Victoza because of the LEADER evidence package, while also weighing how a daily shot fits into that person’s life. Weighing clinical trial evidence and personal habits together helps make the choice feel right, not just medically correct.
6. Saxenda
What if your primary goal is weight management, not glucose control? That’s where Saxenda comes in. Saxenda is liraglutide at a higher dose specifically approved for chronic weight management in adults (and some adolescent indications), whereas Victoza is a lower‑dose liraglutide approved for type 2 diabetes. Semaglutide also has a dedicated high‑dose weight‑loss brand (Wegovy), which is why people often ask if Ozempic and Saxenda are the same — they are related conceptually but not identical.
Saxenda works by reducing appetite and increasing feelings of fullness. In the SCALE trials, liraglutide at the Saxenda dose produced clinically meaningful weight loss for many participants when combined with lifestyle changes. That’s a key point: these medications amplify the effects of diet and activity rather than replacing them.
- Indication: Saxenda is indicated for chronic weight management in adults with BMI criteria or with weight‑related comorbidities. It’s not labeled for diabetes management like Victoza, although some people with diabetes might experience improved glycemic control while using it.
- Administration: Saxenda is a once‑daily injection, and reaching the full therapeutic dose typically requires a gradual dose escalation to improve tolerability.
- Weight loss vs. diabetes meds: Semaglutide (Wegovy) generally leads to greater average weight loss than liraglutide/Saxenda in trials, but individual responses vary. If you’ve tried lifestyle interventions and are exploring pharmacologic options, Saxenda remains a valid and effective choice for many.
- Practical and emotional aspects: Using Saxenda can bring up lots of feelings — relief, hope, frustration. Patients often report a new ability to control portion sizes or resist late‑night snacking, which can shift daily routines and relationships with food. That lived experience is just as important as the numbers on the scale.
Before starting any of these medications, it helps to ask: what are my primary goals (weight, blood sugar, heart health), how do I handle injections, and what supports will I need to maintain lifestyle changes? Weighing those questions with your clinician will point you toward Saxenda, Victoza, Trulicity, semaglutide, or another option — and we can walk through that decision together if you want.
7. Byetta
Curious how an older diabetes medication stacks up against Ozempic? Byetta (exenatide) offers a useful historical and practical contrast. Introduced in the mid-2000s, Byetta is a short-acting GLP-1 receptor agonist given as a twice-daily injection and was one of the first drugs to bring GLP-1 therapy into routine diabetes care.
Imagine you’re starting your day and evening with injections: that’s the rhythm many patients experienced on Byetta, which can mean more planning around meals and timing compared with once-weekly options. Clinical studies from the early development era showed Byetta improved blood sugar control and helped with modest weight loss, but its effects on weight and glycemic control are generally smaller than those seen with newer agents like semaglutide.
Experts commonly point out a few practical considerations:
- Frequency: Twice daily dosing versus Ozempic’s once-weekly schedule — that often matters for adherence.
- Efficacy: Byetta lowers A1c and body weight, but head-to-head and class-wide analyses show semaglutide typically produces greater reductions in both.
- Side effects: Nausea and gastrointestinal upset are common with both, though Byetta’s short-acting profile can mean symptoms are more tied to meal timing. Injection-site reactions are less prominent than with some depot formulations.
If you’ve used Byetta and then tried or are considering semaglutide, you might notice stronger and more sustained appetite suppression and weight loss with semaglutide — a pattern clinicians have observed in practice and that is supported by comparative trial data across the GLP-1 class.
8. Bydureon Bcise
Want a once-weekly option with roots in the same molecule as Byetta? Bydureon BCise is the long-acting, extended-release form of exenatide designed for once-weekly dosing, and it brings a different experience than both twice-daily exenatide and semaglutide.
Think of Bydureon BCise as packaging the same active ingredient in a slow-release vehicle: it smooths exposure over the week rather than spiking around meals. Clinical trial programs (the DURATION-series) demonstrated that weekly exenatide improves A1c and produces modest weight loss compared with placebo and some active comparators. However, when you line it up against semaglutide, the latter generally shows larger average reductions in A1c and body weight in head-to-head or indirect comparisons.
There are a few real-world points people notice:
- Administration: Bydureon BCise uses an autoinjector that’s intended to simplify the weekly injection, but some people report injection-site nodules or lumps because of the depot formulation.
- Effect size: Better than twice-daily exenatide in convenience, but typically less potent for weight loss than semaglutide.
- Side effects: Similar GI profile (nausea, vomiting) plus the possibility of local injection reactions that are specific to extended-release products.
In conversation with endocrinologists, patients who prioritized weekly dosing but had sensitivity to very potent GLP-1 effects sometimes favored Bydureon BCise — it can feel like a gentler weekly option compared with semaglutide’s stronger appetite suppression and metabolic impact.
9. Mounjaro
Ever wondered why some newer diabetes drugs seem to produce dramatically greater weight loss than older GLP-1s? Meet Mounjaro (tirzepatide), a different kind of story. Rather than acting only as a GLP-1 receptor agonist, tirzepatide is a dual GIP and GLP-1 receptor agonist, which gives it a distinctive mechanism and clinical profile.
That dual action is not just a technicality — it translates into real-world differences. The SURPASS trial program showed tirzepatide produced larger reductions in A1c and substantially greater weight loss compared with many comparators, including some direct comparisons with semaglutide. Clinicians often report that patients experience more pronounced appetite suppression and weight changes early on with tirzepatide, though gastrointestinal side effects (nausea, diarrhea) are also common initially.
Key points to consider when comparing Mounjaro to semaglutide:
- Mechanism: Dual GIP/GLP-1 agonism versus semaglutide’s GLP-1-only action — that biological difference helps explain the larger average weight loss seen with tirzepatide.
- Efficacy: Trials (SURPASS series) show tirzepatide often achieves greater A1c and weight reductions than semaglutide, which has led many specialists to consider it when weight loss is a primary goal.
- Side effects & tolerability: Similar GI adverse effects, sometimes more intense early on; clinicians advise slow dose escalation and supportive measures for symptoms.
- Regulatory/indication notes: Both drugs are approved for type 2 diabetes; agents differ in dosing devices, insurance coverage, and emerging guidance on weight-management use.
So when you ask, “Is semaglutide the same as Ozempic?” — Ozempic is semaglutide, but there’s a broader landscape. Mounjaro is a separate class-leading option with a different mechanism and often stronger weight-loss effects. As always, we weigh your goals, tolerability, and practical factors (like cost and dosing preferences) when choosing between them.
10. Zepbound
Have you wondered whether Zepbound is just another name for Ozempic? It’s an easy mix-up — both are injectable drugs that can lead to significant weight loss and improved blood sugar — but Zepbound is not the same as Ozempic. Zepbound is the brand name for tirzepatide, a dual-action medication that stimulates both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP‑1) receptor, while Ozempic contains semaglutide, which targets the GLP‑1 receptor only.
Why does that matter? Mechanistically, combining GIP and GLP‑1 activity with tirzepatide appears to produce additive effects on appetite regulation, insulin release, and metabolic pathways. In clinical research — for example, the SURPASS series of trials — tirzepatide showed larger average reductions in body weight and blood sugar compared with semaglutide in some head‑to‑head and comparator trials. The STEP and SURMOUNT trial families that studied semaglutide and tirzepatide for weight management, respectively, also highlight meaningful differences in average outcomes between the drugs.
From a practical standpoint, both drugs are typically given as weekly injections and can cause similar gastrointestinal side effects (nausea, diarrhea, constipation), but individuals often experience different tolerability profiles. Dosing schedules and titration patterns differ, and insurance coverage or prior authorization rules can make one option more accessible than another.
So when someone asks, “Is Zepbound the same as Ozempic?” the short friend-to-friend answer is: no — they’re different medicines with different mechanisms, and that can mean different results for you depending on your goals and medical history.
How Do I Find Out Which Glp-1 Drug Is Right for Me?
Facing a shelf of brand names and headlines, it’s natural to feel overwhelmed. Let’s break this down into a practical conversation you can have with your clinician — and some steps you can take now.
- Clarify your primary goal. Are you focused on lowering A1c and managing diabetes, achieving substantial weight loss, or both? For example, Ozempic (semaglutide for T2D) and Wegovy (semaglutide for weight) are the same molecule at different doses and approvals; tirzepatide (Zepbound) may produce larger average weight loss in trials. Your goal shapes the recommendation.
- Review your medical history and safety considerations. Tell your clinician about pancreatitis, gallbladder disease, severe gastrointestinal disorders, pregnancy plans, or a personal/family history of medullary thyroid carcinoma or MEN2 — some GLP‑1 drugs carry warnings or contraindications. Your kidney and liver function, other medications, and cardiovascular history also matter.
- Think about side effects and tolerability. GI symptoms are common early on. Some people tolerate one agent better than another; clinicians often start low and titrate slowly to reduce side effects. Ask how side effects are typically managed and what to expect week to week.
- Consider dosing convenience and preferences. All these medications are usually weekly injections, but titration schedules differ. If you prefer fewer dose changes or a specific pen device, say so — some devices feel easier to use for certain people.
- Check cost and coverage. Insurance coverage varies by indication (diabetes vs. chronic weight management) and by brand. Prior authorizations are common. Ask your provider’s office to check coverage early — sometimes they suggest an accessible option first.
- Look at the evidence for your situation. Ask whether there are head‑to‑head data for people like you (for example, people with type 2 diabetes, with or without obesity). Studies like the STEP trials (semaglutide) and SURPASS/SURMOUNT trials (tirzepatide) provide helpful context about average results and safety, but individual responses vary.
- Plan for lifestyle support and monitoring. Medication is part of a plan: nutrition, physical activity, behavioral support, and follow-up labs matter. Ask how often you’ll be monitored and what targets you should expect.
- Build in a trial period and reassessment. Many clinicians consider a 12–16 week window to assess tolerability and early response, with adjustments after that. If side effects or results aren’t acceptable, there are other options.
Here are some practical questions to bring to your appointment:
- “Based on my goals, which drug do you recommend and why?”
- “What side effects should I expect, and how will we manage them?”
- “How long before I’ll see results, and when will we reassess?”
- “Are there safety concerns for me (pregnancy, pancreatitis, thyroid issues)?”
- “Who handles insurance approvals and cost‑saving options?”
Clinicians — especially endocrinologists, obesity medicine specialists, and experienced primary care physicians — will weigh clinical evidence, your preferences, and coverage realities. If you ever feel unsure, asking for a second opinion or referral to a specialist is reasonable; we all deserve a plan that fits our life.
The Bottom Line
Let’s keep it simple and honest. Semaglutide is the active molecule; Ozempic is a brand of semaglutide used for type 2 diabetes (and Wegovy is semaglutide at a higher dose for weight management). Zepbound (tirzepatide) is a different drug — a dual GIP/GLP‑1 agonist — and it’s not the same as semaglutide or Ozempic. The differences in mechanism translate into differences in average efficacy, side effects, dosing, and real‑world access.
Which one is right for you depends on your health goals, medical history, how you tolerate side effects, and what your insurance will cover. Talk with your clinician, bring the questions above, and remember: medication is one tool in a broader plan that includes lifestyle support and ongoing follow‑up. Want help putting together questions for your provider or comparing specific pros and cons for your situation? I’m here to help.
References
Curious which studies and expert sources shaped what we just discussed? Let’s walk through the evidence together — I’ve pulled authoritative trials, regulatory documents, reviews, and guideline statements so you can see exactly where claims about semaglutide and Ozempic come from. Reading the references can help you separate the brand-name headlines from the underlying drug science.
View All References (18)
- Ozempic (semaglutide) Prescribing Information — Novo Nordisk. The official FDA label that outlines approved uses, dosing, safety warnings, and clinical trial summaries behind the brand Ozempic.
- Wegovy (semaglutide 2.4 mg) Prescribing Information — Novo Nordisk. The FDA label for the higher-dose semaglutide formulation approved specifically for chronic weight management, useful for comparing indications and dosing.
- SUSTAIN Program trials (semaglutide for type 2 diabetes). A series of randomized clinical trials that evaluated efficacy and safety of subcutaneous semaglutide (the basis for Ozempic’s diabetes indication), including glycemic control and weight effects.
- SUSTAIN-6 (cardiovascular outcomes trial). A large outcomes study assessing major adverse cardiovascular events with semaglutide in people with type 2 diabetes — important context for cardiovascular risk discussions.
- STEP Program trials (semaglutide 2.4 mg for weight loss). Randomized trials that demonstrated substantial weight loss with once‑weekly semaglutide at obesity-management doses (the evidence underpinning Wegovy).
- SELECT cardiovascular outcomes trial. A major outcomes trial examining whether semaglutide for weight management reduces cardiovascular events in people with overweight or obesity and cardiovascular disease.
- Comparative pharmacology and formulation reviews. Articles and reviews that explain how the same active molecule (semaglutide) is formulated differently across Ozempic, Wegovy, and oral semaglutide (Rybelsus), affecting dosing and approved uses.
- Meta-analyses of GLP-1 receptor agonists. Systematic reviews pooling data across GLP-1 drugs that help us understand class effects (weight loss, glucose lowering, cardiovascular effects) and differences among agents.
- Guideline statements from ADA and AACE. Professional society recommendations describing when GLP‑1 receptor agonists (including semaglutide) are appropriate for diabetes and weight management.
- Real-world observational studies. Registry and claims-based analyses showing how semaglutide performs outside clinical trials and patterns of use with brand products.
- Safety signal and adverse event reviews. Studies and reviews that examined gastrointestinal side effects, pancreatitis signal, gallbladder disease, and rare but important safety considerations.
- Preclinical studies on thyroid C‑cell findings. Animal data that informed boxed warnings and regulatory discussion about rodent thyroid C‑cell tumors and how that translates to human risk assessment.
- Pharmacokinetic and pharmacodynamic studies. Trials that detail absorption, half-life, and dose–response for subcutaneous semaglutide formulations — useful for understanding why dosing differs between products.
- Oral semaglutide (Rybelsus) clinical trials and reviews. Evidence showing how oral semaglutide differs in formulation and administration from injectable forms, even though the active molecule is the same.
- Health-economics and access studies. Analyses of cost, insurance coverage, and the real-world access challenges patients face when seeking brand-name formulations.
- Patient-facing educational materials and clinician summaries. Concise, practical summaries used in clinics to explain differences between brand names and generic molecules.
- Expert commentaries and editorials. Endocrinologists and cardiologists interpreting trial results and regulatory decisions, helping clinicians translate evidence into practice.
- Cochrane-style evidence syntheses on GLP‑1s for weight and diabetes. High-quality reviews that critically appraise trial design, bias risk, and strength of evidence across the drug class.
Article Resources
Want to know how I chose and used these resources? Here’s the method and some practical next steps so you can evaluate evidence alongside me.
- Why these types of sources matter: Regulatory labels (like FDA summaries) give authoritative, vetted statements about approved uses and risks. Randomized controlled trials (SUSTAIN, STEP, SELECT) provide the highest-quality evidence of benefit and harm. Meta-analyses and guidelines synthesize that evidence so clinicians can make real-world decisions.
- How to read a trial summary: Focus first on the population studied (did they have diabetes, obesity, or both?), the dose used (Ozempic vs. Wegovy doses differ), primary outcomes (A1c, weight, cardiovascular events), and length of follow-up. That context explains whether a result applies to you or someone you care for.
- Limitations and common caveats: Trials are done under controlled conditions and with selected participants; real-world adherence, insurance coverage, and side-effect profiles can alter outcomes. That’s why observational studies and guideline commentary are included — they help bridge trial results to everyday practice.
- Practical resources clinicians use: Prescribing information, clinical guideline summaries, and patient leaflets — these are the tools clinicians reference during conversations about risks, benefits, and cost considerations.
- Questions you might ask your clinician: Which semaglutide formulation matches my goals (glucose control vs. weight loss)? How does the dose compare to what was tested? What side effects should I expect and how long will they last?
- Transparency note: Where a trial or regulatory document informed a specific statement in the article, I weighted those sources most heavily. For broader context, I used reviews and guideline statements to interpret how the evidence is applied in practice.
- Want the primary papers summarized? If you’d like, I can provide concise takeaways for any of the trials mentioned (SUSTAIN-6, STEP 1, SELECT, etc.) — including the question they asked, how they did it, the main results, and what it means for someone like you.
Which reference would you like to dive into first — the diabetes trials, the weight-loss studies, or the safety and regulatory documents? We can open any one of them together and I’ll summarize the key parts in plain language.
Frequently Asked Questions
Have you ever wondered whether the medicine in your prescription is exactly the same as the one a friend gets from a different source? You’re not alone — many people are confused about the difference between a branded drug like Ozempic and generic or compounded forms of the same active ingredient, semaglutide. Below we unpack the most common questions, blending real-world examples, clinical evidence, and practical steps you can take.
- Q: Is Ozempic the same thing as semaglutide?A: Ozempic is a brand name product that contains semaglutide, the active GLP‑1 receptor agonist used to treat type 2 diabetes and often associated with weight loss. The medication works the same way at the molecular level — semaglutide binds GLP‑1 receptors to stimulate insulin release, slow gastric emptying, and reduce appetite — but the branded product includes a specific formulation, device (pre-filled pen), and manufacturing quality controls that are regulated and approved by health authorities.
- Q: What is compounded semaglutide?A: Compounded semaglutide is made by a compounding pharmacy that mixes or repackages semaglutide into forms or doses not commercially available. People often turn to compounded versions for cost savings or availability, but these products are not subject to the same FDA approval pathway as Ozempic and may vary in potency, sterility, and delivery format.
- Q: Will a compounded product work as well as Ozempic?A: It depends. The active ingredient can be the same, and many compounding pharmacies follow strict sterile procedures, but clinical trials that established the safety and efficacy of semaglutide used branded, regulated products (for example, the SUSTAIN trials for diabetes and the STEP trials for weight management). Those trials used specific doses and delivery methods — differences in formulation, purity, or dosing can affect real-world outcomes.
- Q: Are side effects the same?A: The side-effect profile of semaglutide — nausea, vomiting, diarrhea, constipation, and potential risk of hypoglycemia when combined with other diabetes medicines — is tied to the drug itself. However, compounding introduces additional safety concerns such as contamination, inaccurate dosing, or particulate matter that could cause injection site reactions or infections.
- Q: Is compounded semaglutide legal and safe?A: Compounding is legal when performed within state and federal rules, but it is a different regulatory pathway. State boards and the FDA have issued advisories cautioning clinicians and patients about off-label compounding of drugs that are otherwise available commercially, especially when it bypasses approved manufacturing processes. “Legal” does not automatically mean “identical quality.”
- Q: How should I choose between Ozempic and a compounded option?A: Ask questions: who prepared the product, can they provide a certificate of analysis, what sterility and potency testing was done, what is the beyond‑use date, and is the pharmacy accredited (for sterile compounding, look for USP 797 compliance and board certifications)? Also discuss cost, insurance coverage, and clinical follow-up with your prescriber. Many clinicians recommend branded formulations when possible because they match the data used to guide dosing and monitoring.
- Q: What about storage and administration differences?A: Branded Ozempic comes in a prefilled pen with clear storage and dosing instructions. Compounded semaglutide may be dispensed in vials or syringes requiring different handling — refrigeration, light protection, and strict aseptic technique for drawing doses. These practical differences can affect safety and convenience.
Frequently Asked Questions About Compounded Semaglutide and Ozempic
Curious about the finer points — like why two vials labeled “semaglutide” can feel like different products? Let’s walk through details clinicians and pharmacists want you to know, plus signs that a compounded product meets reasonable quality expectations.
- Are they interchangeable in a prescription?No — interchangeability depends on state law, prescriber intent, and pharmacy practice. If your prescriber writes “dispense as written” for Ozempic, a pharmacist should not substitute a compounded product without consent. Even when substitution is permitted, dosing equivalence and device differences mean you and your clinician need to confirm a safe plan for switching.
- How do clinical trials inform what we expect from Ozempic?Large randomized trials such as the SUSTAIN series (for diabetes) and STEP trials (for weight loss with semaglutide formulations) provide the evidence clinicians use to choose doses and monitor outcomes. Those studies assessed specific formulations and devices under controlled conditions — the outcomes reported (A1c reduction, weight loss, adverse events) reflect those regulated products.
- What are the main risks of choosing a compounded product?Beyond the expected pharmacologic side effects, risks include variable potency (under- or overdosing), sterility breaches, contamination, and lack of batch testing. There have been notable historical cases where compounded sterile products led to infections because of lapses in aseptic technique — a reminder that sterility matters for injected drugs.
- How can you verify a compounding pharmacy?Look for accreditation from recognized organizations, documentation of quality control, and the ability to provide a certificate of analysis. Ask whether the pharmacy follows USP standards for compounded sterile preparations (often referenced as USP 797) and if they perform sterility and potency testing on batches. Speak to the pharmacist about beyond‑use dating and return policies.
- Will insurance cover compounded semaglutide?Often not. Branded products like Ozempic may be covered by Medicare or commercial plans (with prior authorization), whereas compounded products are frequently out-of-pocket. That cost difference is a major reason patients consider compounding, but it comes with trade-offs in oversight and documented quality.
- Practical example:Imagine two patients: one uses Ozempic pens with routine follow-ups and predictable dose increments; the other orders compounded vials because of cost, stores them in a home freezer then thaws and draws doses manually. The second patient has a higher risk of dosing error, but may save money. Real-world experiences like these are why shared decision-making with your clinician matters.
How We Reviewed This Article:
You’re probably wondering how reliable this information is — we treated this topic like a conversation between you and clinicians who want clarity.
- Scope and sources: We reviewed peer-reviewed clinical trial summaries (including major semaglutide trials used to establish safety and efficacy for diabetes and weight management), prescribing information for branded semaglutide products, FDA and state pharmacy board advisories about compounding, and professional guidance on sterile compounding standards. We also considered coverage trends from payer literature and real-world practice notes from endocrinologists and clinical pharmacists.
- Expert input: We synthesized common viewpoints from endocrinologists and pharmacists: clinicians emphasize using regulated, approved products when possible because trial data and labeled dosing apply directly; pharmacists highlight the importance of sterility, potency testing, and documented quality for any compounded sterile preparation.
- Quality checks: We cross‑checked safety signals and common adverse effects against trial data and drug labels, and we flagged regulatory differences between FDA‑approved products and compounded preparations as a core theme. Where empirical data comparing branded vs compounded semaglutide are sparse, we noted that evidence gap rather than speculated.
- Limitations: The landscape for GLP‑1 treatments and compounding practices evolves quickly — new formulations, supply shortages, or regulatory updates can change availability and guidance. We did not test individual compounding pharmacies, so we cannot certify particular vendors; instead, we offer practical questions and red flags you can use to evaluate a pharmacy or product.
- Conflicts of interest: We disclose that this write-up is informational and not an endorsement of any product, pharmacy, or brand. Decisions should be made with your prescriber, considering your health history, insurance, and access.
- How you can use this: Take these talking points to your clinician or pharmacist: ask about the exact product name, request documentation of testing for compounded products, verify storage and administration instructions, and confirm follow-up monitoring plans. If cost is the driver, discuss assistance programs or authorized alternatives before choosing a compounded route.
Summary
Have you ever heard someone say “semaglutide” and “Ozempic” like they’re interchangeable and wondered if that’s true? You’re not alone—it’s a common question that matters if you’re managing diabetes, exploring weight-loss options, or just trying to understand a prescription. Let’s clear this up in a friendly, practical way.
Semaglutide is the name of the active drug—the molecule itself, a glucagon-like peptide-1 (GLP-1) receptor agonist. Ozempic is one of the brand-name products that contains semaglutide, formulated and dosed specifically by its manufacturer for the treatment of type 2 diabetes. Think of semaglutide as the ingredient and Ozempic as a particular recipe using that ingredient.
That distinction matters because the same active molecule can appear in different products with different purposes, doses, and ways to take it:
- Ozempic – injectable, once-weekly, approved primarily for type 2 diabetes management; common starter doses and titration schedules differ from weight-loss formulations.
- Wegovy – also injectable and contains semaglutide, but formulated and dosed (higher maintenance dose) specifically for chronic weight management and obesity treatment.
- Rybelsus – oral semaglutide, taken daily, used for type 2 diabetes; same molecule but a different delivery system and dosing approach.
Mechanistically, semaglutide works by enhancing insulin secretion when glucose is high, slowing gastric emptying, and reducing appetite through brain pathways—effects that explain why it helps with blood sugar control and, at higher doses, substantial weight loss. Clinical trials back this up: diabetes-focused trials (the SUSTAIN series) showed meaningful HbA1c and weight reductions with semaglutide-containing products, and obesity-focused trials (the STEP series) found large average weight losses at the higher, weight-management dose used in Wegovy.
But same molecule doesn’t always mean same experience. Differences to keep in mind:
- Dose and target: Ozempic dosing for diabetes (commonly starting low and titrating to modest weekly doses) is different from Wegovy’s higher maintenance dose for weight loss.
- Formulation and route: injectable weekly pens versus daily oral tablets change convenience, absorption, and the way insurance may cover them.
- Indication and approval: doctors prescribe Ozempic for type 2 diabetes; Wegovy is the approved branded semaglutide for obesity—using one for the other’s indication may be off-label and handled differently by insurers.
- Side effects and monitoring: GI side effects (nausea, vomiting, diarrhea) are common across formulations; there are class warnings (e.g., about thyroid C-cell tumors in rodent studies and certain personal/family histories like medullary thyroid carcinoma or MEN2) that we discuss with our clinicians.
If you’re weighing whether a semaglutide product is right for you, ask these practical questions:
- What is the specific brand and dose being prescribed, and why that one?
- Is the goal blood-sugar control, weight loss, or both?
- How will dosing, administration, cost, and insurance coverage affect adherence?
- Are there medical reasons (family history, pancreatitis, pregnancy plans) that change the risk–benefit balance?
In short: semaglutide is the drug; Ozempic is a brand that contains semaglutide. They’re related, but not identical in how you’ll use them, what they’re approved for, and what to expect. Weigh the clinical evidence, practical considerations, and your personal goals with your healthcare provider so you choose the right formulation and plan for you.


